Diastereomers are stereoisomers that are not mirror images of each other and differ in the configuration of at least one chiral center. These molecules exhibit distinct physical and chemical properties, influencing their behavior in chemical reactions and biological systems. Explore the rest of the article to understand how diastereomers impact stereochemistry and practical applications.
Table of Comparison
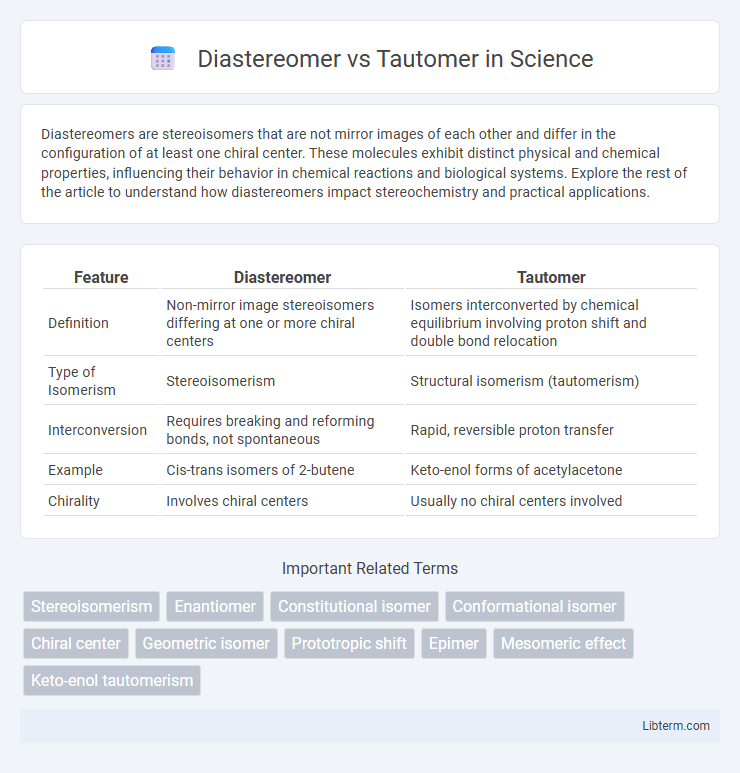
| Feature | Diastereomer | Tautomer |
|---|---|---|
| Definition | Non-mirror image stereoisomers differing at one or more chiral centers | Isomers interconverted by chemical equilibrium involving proton shift and double bond relocation |
| Type of Isomerism | Stereoisomerism | Structural isomerism (tautomerism) |
| Interconversion | Requires breaking and reforming bonds, not spontaneous | Rapid, reversible proton transfer |
| Example | Cis-trans isomers of 2-butene | Keto-enol forms of acetylacetone |
| Chirality | Involves chiral centers | Usually no chiral centers involved |
Introduction to Diastereomers and Tautomers
Diastereomers are stereoisomers that are not mirror images of each other and have different physical and chemical properties due to the presence of multiple chiral centers. Tautomers are isomers that readily interconvert through chemical reactions, typically involving proton transfer and relocation of double bonds, exemplified by keto-enol tautomerism. Understanding the distinction between diastereomers and tautomers is essential for studying molecular stereochemistry and dynamic chemical equilibria.
Defining Diastereomers: Structure and Properties
Diastereomers are stereoisomers that have multiple chiral centers and differ in the spatial arrangement of atoms at one or more, but not all, of these centers, resulting in distinct physical and chemical properties. Unlike enantiomers, diastereomers are not mirror images and often display different melting points, boiling points, and reactivity. Their diverse stereochemistry impacts biological activity and synthesis pathways, making diastereomers critical in pharmaceuticals and asymmetric synthesis.
Understanding Tautomers: Concepts and Mechanisms
Tautomers are isomers that interconvert through the relocation of a proton and a shift in a double bond, typically involving keto-enol or imine-enamine pairs, which distinguishes them from diastereomers that differ in spatial arrangement without such proton shifts. The tautomerization mechanism is often acid- or base-catalyzed, facilitating proton transfer and bond rearrangement to achieve equilibrium between forms, significantly influencing chemical reactivity and biological activity. Understanding tautomerism is crucial in fields like medicinal chemistry and enzymology, where tautomeric states affect molecular recognition and function.
Key Differences Between Diastereomers and Tautomers
Diastereomers are stereoisomers with multiple chiral centers that differ in configuration at one or more (but not all) stereocenters, resulting in distinct spatial arrangements and physical properties. Tautomers are structural isomers that rapidly interconvert through the migration of a proton and a shift in double bonds, commonly observed as keto-enol pairs with differing connectivity rather than configuration. The critical difference lies in diastereomers being stereoisomers with stable, fixed configurations, whereas tautomers are dynamic constitutional isomers in equilibrium that differ in bonding and proton position.
Methods for Identifying Diastereomers vs Tautomers
Diastereomers can be identified using techniques such as nuclear magnetic resonance (NMR) spectroscopy, which distinguishes differences in chemical shifts due to their distinct spatial arrangements, and chiral chromatography that separates stereoisomers based on varying interactions with chiral stationary phases. Tautomers are primarily detected through methods like infrared (IR) spectroscopy, which captures characteristic shifts in functional group vibrations, and UV-visible spectroscopy, highlighting changes in conjugation patterns; additionally, NMR can observe the dynamic equilibrium between tautomeric forms. Mass spectrometry provides complementary information by confirming molecular formulas without distinguishing stereoisomers or tautomers directly.
Importance of Stereochemistry in Diastereomers
Diastereomers exhibit different spatial arrangements of atoms, which significantly influence their chemical and physical properties, making stereochemistry crucial for understanding their distinct biological activities and reactivity. Unlike tautomers, which interconvert through proton shifts and primarily differ in connectivity rather than spatial configuration, diastereomers maintain fixed stereochemical differences that affect drug efficacy, enzyme interaction, and material properties. Precise control of stereochemistry in diastereomers is essential in pharmaceuticals and synthetic chemistry to achieve desired stereoselectivity and functional outcomes.
Role of Prototropic Shifts in Tautomerism
Tautomerism involves prototropic shifts where a proton relocates within a molecule, resulting in isomers called tautomers with distinct connectivity and properties. Diastereomers, in contrast, are stereoisomers that differ in spatial arrangement without proton transfer, maintaining the same connectivity. Prototropic shifts play a crucial role in tautomerism by enabling dynamic equilibrium between tautomers, affecting chemical reactivity and biological activity.
Examples of Diastereomers and Tautomers in Organic Chemistry
Diastereomers include examples such as glucose and galactose, which differ in the configuration at one or more stereocenters but are not mirror images, while tautomers commonly occur in keto-enol forms like acetone (keto form) and its enol tautomer. Another example of diastereomers is cis- and trans-2-butene, exhibiting different spatial arrangements of substituents around the double bond. In tautomerism, compounds like uracil undergo rapid proton shifts between keto and enol forms, influencing their chemical behavior in biological systems.
Applications in Pharmaceuticals and Chemical Synthesis
Diastereomers are crucial in pharmaceuticals due to their distinct physical and chemical properties, influencing drug efficacy, stability, and pharmacokinetics, thus enabling targeted drug design and development. Tautomers play a significant role in chemical synthesis by affecting reaction mechanisms and product distribution, particularly in nucleic acid chemistry and heterocyclic compound formation. Understanding the dynamics between diastereomeric and tautomeric forms aids in optimizing synthetic pathways and improving pharmaceutical formulations.
Summary: Choosing Between Diastereomer and Tautomer Analysis
Diastereomers are stereoisomers with different spatial arrangements but the same molecular formula, often affecting physical properties and biological activity, while tautomers are isomers that interconvert through proton shifts, primarily influencing chemical reactivity and equilibrium states. Selecting between diastereomer and tautomer analysis depends on the study's goal: diastereomer analysis is critical for stereochemical configuration and chirality assessment, whereas tautomer analysis is essential for understanding dynamic equilibrium and chemical behavior under varying conditions. Emphasizing the correct isomer type enhances accuracy in drug design, synthesis, and molecular characterization.
Diastereomer Infographic
 libterm.com
libterm.com