Isoosmotic solutions have the same osmotic pressure as bodily fluids, ensuring that there is no net movement of water across cell membranes, which prevents cells from swelling or shrinking. This balance is critical for maintaining cellular integrity and function in medical treatments such as intravenous therapy. Discover how isoosmotic conditions impact your body's cells and why they matter in healthcare by reading the full article.
Table of Comparison
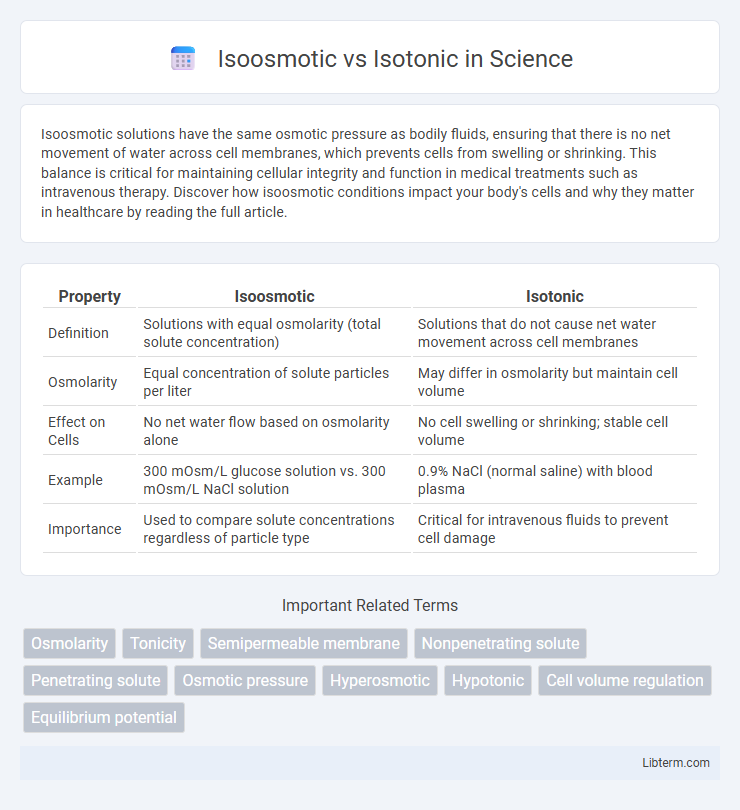
| Property | Isoosmotic | Isotonic |
|---|---|---|
| Definition | Solutions with equal osmolarity (total solute concentration) | Solutions that do not cause net water movement across cell membranes |
| Osmolarity | Equal concentration of solute particles per liter | May differ in osmolarity but maintain cell volume |
| Effect on Cells | No net water flow based on osmolarity alone | No cell swelling or shrinking; stable cell volume |
| Example | 300 mOsm/L glucose solution vs. 300 mOsm/L NaCl solution | 0.9% NaCl (normal saline) with blood plasma |
| Importance | Used to compare solute concentrations regardless of particle type | Critical for intravenous fluids to prevent cell damage |
Introduction to Isoosmotic and Isotonic Concepts
Isoosmotic solutions have equal osmotic pressure due to the same total concentration of solutes, regardless of the solute type, whereas isotonic solutions specifically prevent water movement across cell membranes by matching solute concentrations that affect cell volume. Isoosmotic conditions are defined by osmolarity, measured in osmoles per liter, reflecting total solute particles in solution. Isotonicity is crucial for maintaining cell integrity and function during medical treatments, relying on solute types that do not cause osmotic water shifts across semi-permeable membranes.
Defining Isoosmotic Solutions
Isoosmotic solutions contain the same total concentration of solute particles per unit volume as the reference solution, regardless of the solute type or its dissociation. These solutions balance osmotic pressure by maintaining equal osmolarity, preventing net water movement across semipermeable membranes. Unlike isotonic solutions, which specifically avoid cell volume changes, isoosmotic solutions emphasize equal solute particle concentration without necessarily ensuring cell integrity.
Understanding Isotonic Solutions
Isotonic solutions have the same osmolarity as the cells or body fluids to which they are compared, ensuring no net movement of water across cell membranes and maintaining cellular hydration and volume. In contrast, isoosmotic solutions have equal osmotic concentrations but may differ in tonicity if the solutes do not cross the membrane freely, potentially causing water movement. Understanding isotonic solutions is critical in medical applications like intravenous therapy, where maintaining cell integrity and fluid balance is essential.
Key Differences Between Isoosmotic and Isotonic
Isoosmotic solutions have equal osmolarity to another solution, meaning they contain the same total concentration of solutes per liter, while isotonic solutions prevent water movement across a membrane to avoid cell swelling or shrinking. The key difference lies in their biological effect: isoosmotic solutions may not be isotonic if the solutes can cross the membrane, whereas isotonic solutions maintain cell volume by ensuring no net water flow. Isoosmotic solutions focus on solute concentration equality, isotonic solutions emphasize osmotic pressure balance relative to cells.
Mechanisms of Osmosis and Tonicity
Isoosmotic solutions have the same total solute concentration as the intracellular fluid, ensuring no net movement of water across the cell membrane through osmosis. Isotonic solutions maintain cell volume by having equal osmotic pressure, preventing water from entering or leaving the cell. Osmosis drives water movement based on solute concentration gradients, while tonicity depends specifically on solutes that cannot cross the membrane, affecting cell volume indirectly.
Physiological Importance in Medical Practice
Isoosmotic and isotonic solutions play critical roles in medical practice by maintaining cellular integrity during fluid administration; isoosmotic solutions have equal osmolarity to body fluids, preventing water movement across cell membranes, while isotonic solutions match the extracellular fluid tonicity, avoiding cell swelling or shrinkage. The physiological importance lies in their use for intravenous therapy, where isoosmotic solutions are ideal for balancing osmotic pressure without disrupting cell volume, and isotonic solutions ensure stable fluid balance in the vascular compartment. Precise selection between these solutions prevents complications such as cellular edema or dehydration, crucial for patient safety in treatments like rehydration and electrolyte management.
Examples of Isoosmotic Solutions
Isoosmotic solutions have the same osmolarity as the reference solution but may differ in solute composition; examples include 300 mOsm/kg urea solution and 300 mM glucose solution matching plasma osmolarity. Isotonic solutions, like 0.9% saline (154 mM NaCl), maintain cell volume by having equal osmotic pressure and do not cause water movement across cell membranes. Isoosmotic solutions can vary in tonicity if solutes freely cross membranes, whereas isotonic solutions prevent net water flow, preserving cell integrity.
Examples of Isotonic Solutions
Isotonic solutions have the same solute concentration as cells, preventing osmosis that would cause cells to shrink or swell. Examples of isotonic solutions include 0.9% sodium chloride (normal saline), lactated Ringer's solution, and 5% dextrose in water (D5W) after metabolism, all commonly used in intravenous therapy to maintain fluid balance. These solutions are essential for medical treatments that require safe fluid replacement without disrupting cellular function.
Clinical Implications and Applications
Isoosmotic solutions have the same osmolarity as plasma but may differ in solute composition, while isotonic solutions have equal osmotic pressure preventing net water movement across cell membranes; this distinction is critical for intravenous fluid therapy to avoid cellular swelling or shrinkage. In clinical settings, isotonic fluids like 0.9% saline are preferred for volume replacement to maintain extracellular fluid balance without disrupting intracellular osmolarity. Understanding both concepts guides the selection of appropriate fluids in managing conditions such as dehydration, hyponatremia, and edema, optimizing patient outcomes and preventing complications associated with fluid shifts.
Summary: Isoosmotic vs Isotonic Explained
Isoosmotic solutions have the same total solute concentration as another solution, regardless of solute type or effect on cells, while isotonic solutions specifically prevent net water movement across cell membranes, maintaining cell volume. Isoosmotic conditions are defined by equal osmolarity, but they may not always be isotonic if the solutes can freely cross or affect cell integrity. Understanding the distinction is essential in medical and biological contexts to ensure appropriate fluid balance and avoid cellular damage.
Isoosmotic Infographic
 libterm.com
libterm.com