Amphoteric substances can act as both acids and bases depending on the environment, making them versatile in chemical reactions. This dual nature is critical in processes like buffer systems and industrial applications. Explore the rest of the article to understand how amphoteric behavior impacts your daily life and various scientific fields.
Table of Comparison
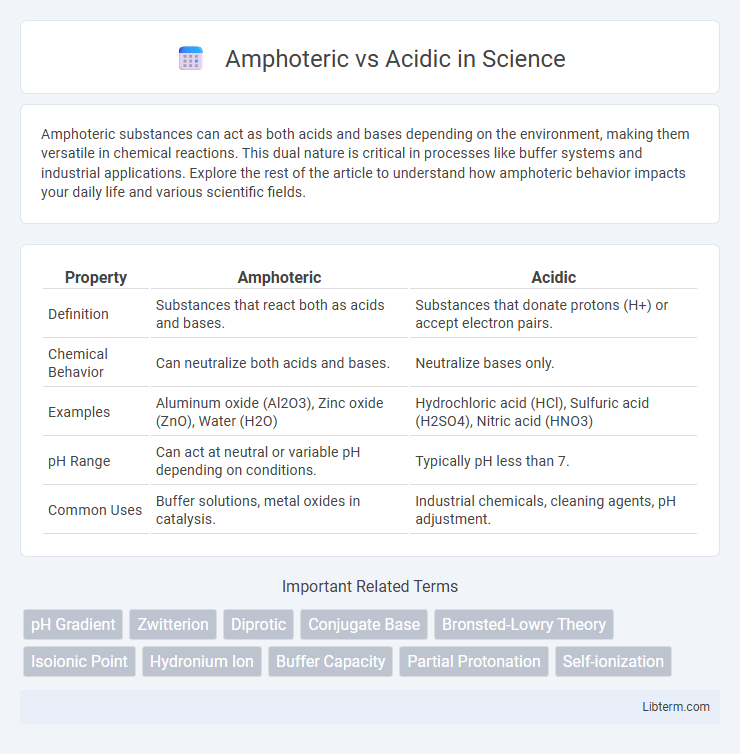
| Property | Amphoteric | Acidic |
|---|---|---|
| Definition | Substances that react both as acids and bases. | Substances that donate protons (H+) or accept electron pairs. |
| Chemical Behavior | Can neutralize both acids and bases. | Neutralize bases only. |
| Examples | Aluminum oxide (Al2O3), Zinc oxide (ZnO), Water (H2O) | Hydrochloric acid (HCl), Sulfuric acid (H2SO4), Nitric acid (HNO3) |
| pH Range | Can act at neutral or variable pH depending on conditions. | Typically pH less than 7. |
| Common Uses | Buffer solutions, metal oxides in catalysis. | Industrial chemicals, cleaning agents, pH adjustment. |
Introduction to Amphoteric and Acidic Substances
Amphoteric substances can react both as acids and bases due to their ability to donate or accept protons, making them versatile in chemical reactions. Acidic substances release hydrogen ions (H+) in aqueous solutions, contributing to their characteristic sour taste and corrosive properties. Understanding the distinct behaviors of amphoteric and acidic substances is essential in fields such as chemistry, biochemistry, and environmental science.
Defining Amphoteric Compounds
Amphoteric compounds exhibit the unique ability to react both as acids and bases depending on the chemical environment, differentiating them from purely acidic substances that solely donate protons in reactions. These compounds, such as aluminum hydroxide and zinc oxide, contain active sites that can either accept or donate protons, enabling versatile reactivity in acid-base chemistry. The dual functionality of amphoteric substances plays a crucial role in various chemical processes, including buffering, neutralization, and catalysis, making their behavior distinct from strictly acidic compounds.
Understanding Acidic Substances
Acidic substances release hydrogen ions (H+) in aqueous solutions, lowering the pH below 7 and exhibiting properties such as sour taste, electrical conductivity, and reactivity with bases to form salts and water. Common examples include hydrochloric acid (HCl) and sulfuric acid (H2SO4), which are strong acids fully dissociating in water. Understanding acidic behavior is essential in chemical reactions, industrial processes, and biological systems due to their role in pH regulation and reactivity.
Chemical Properties: Amphoteric vs Acidic
Amphoteric substances can react both as acids and bases, exhibiting dual chemical behavior depending on the environment, whereas acidic substances exclusively donate protons (H+ ions) in reactions. Amphoteric compounds like aluminum hydroxide (Al(OH)3) neutralize both acids and bases, while acids such as hydrochloric acid (HCl) consistently increase hydrogen ion concentration in aqueous solutions. The chemical property distinction lies in amphoterism's ability to engage in proton transfer bidirectionally, contrasted with the unidirectional proton donation characteristic of acidic behavior.
Common Examples of Amphoteric Compounds
Aluminum hydroxide, zinc oxide, and beryllium hydroxide are common amphoteric compounds, reacting with both acids and bases to form salts and water. Amphoteric oxides and hydroxides like aluminum oxide and zinc hydroxide exhibit dual behavior by neutralizing hydrochloric acid (acidic reaction) and sodium hydroxide (basic reaction). These compounds contrast with purely acidic substances such as sulfuric acid or nitric acid, which only donate protons in chemical reactions.
Everyday Examples of Acidic Substances
Common acidic substances encountered daily include lemon juice, vinegar, and aspirin, each containing natural or synthetic acids like citric acid, acetic acid, and acetylsalicylic acid. These acids release hydrogen ions (H+) in aqueous solutions, contributing to their sour taste and ability to react with bases. Understanding the properties of these acidic substances is essential for applications in cooking, cleaning, and medicine, highlighting their practical significance in everyday life.
Importance in Chemical Reactions
Amphoteric substances, capable of acting as both acids and bases, play a crucial role in chemical reactions by stabilizing pH and enabling versatile reaction pathways. Acidic compounds donate protons, driving reactions forward and facilitating processes such as catalysis and synthesis. Understanding the balance between amphoteric and acidic behavior is essential for optimizing reaction conditions in industrial and biological systems.
Key Differences: Amphoteric vs Acidic
Amphoteric substances can react both as acids and bases depending on the environment, whereas acidic substances exclusively donate protons (H+ ions) in chemical reactions. The key difference lies in amphoterics like aluminum hydroxide, which neutralize acids and bases, while acidic compounds like hydrochloric acid solely increase hydrogen ion concentration in solutions. Understanding this distinction is crucial for applications in chemical synthesis, environmental science, and industrial processes.
Real-world Applications and Uses
Amphoteric substances, such as aluminum hydroxide and zinc oxide, play a critical role in water treatment and pharmaceutical formulations due to their ability to react with both acids and bases, enabling neutralization and adsorption processes. Acidic compounds, including sulfuric acid and hydrochloric acid, are extensively utilized in industrial manufacturing, metal processing, and chemical synthesis for pH control and etching applications. Understanding the distinct reactivity of amphoteric versus acidic materials guides their selection in environmental remediation, waste management, and manufacturing sectors to optimize performance and safety.
Conclusion: Choosing Between Amphoteric and Acidic Substances
Selecting between amphoteric and acidic substances depends on the specific chemical reactions and environmental conditions involved. Amphoteric substances offer versatility by reacting as either acids or bases, making them ideal for neutralization and buffering applications. Acidic substances are preferred when a strong proton donor is required, especially in reactions necessitating lower pH or corrosion processes.
Amphoteric Infographic
 libterm.com
libterm.com