Filtration is a critical process used to remove impurities and solid particles from liquids or gases, enhancing the quality and safety of the final product. It plays a vital role in various industries such as water treatment, pharmaceuticals, and food production by ensuring contaminants are effectively separated. Explore the rest of the article to understand how advanced filtration techniques can improve your applications and outcomes.
Table of Comparison
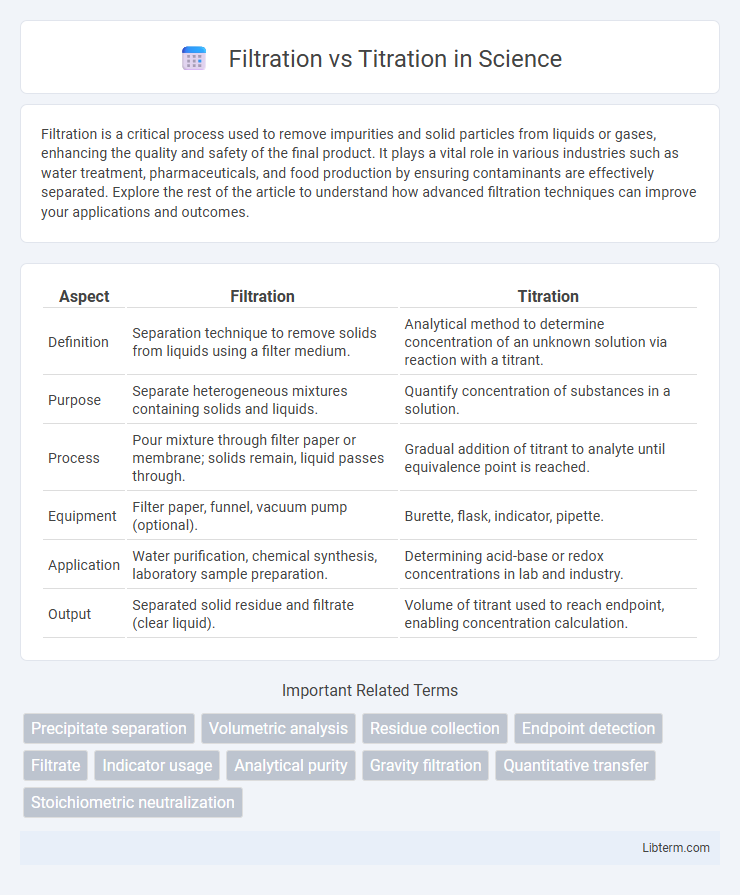
| Aspect | Filtration | Titration |
|---|---|---|
| Definition | Separation technique to remove solids from liquids using a filter medium. | Analytical method to determine concentration of an unknown solution via reaction with a titrant. |
| Purpose | Separate heterogeneous mixtures containing solids and liquids. | Quantify concentration of substances in a solution. |
| Process | Pour mixture through filter paper or membrane; solids remain, liquid passes through. | Gradual addition of titrant to analyte until equivalence point is reached. |
| Equipment | Filter paper, funnel, vacuum pump (optional). | Burette, flask, indicator, pipette. |
| Application | Water purification, chemical synthesis, laboratory sample preparation. | Determining acid-base or redox concentrations in lab and industry. |
| Output | Separated solid residue and filtrate (clear liquid). | Volume of titrant used to reach endpoint, enabling concentration calculation. |
Introduction to Filtration and Titration
Filtration is a physical separation technique used to separate solids from liquids or gases by passing the mixture through a porous medium, commonly filter paper or a membrane. Titration is a quantitative analytical method used to determine the concentration of an unknown solution by reacting it with a solution of known concentration, often using indicators to signal the endpoint. Both filtration and titration are fundamental laboratory procedures in chemistry, serving different purposes--filtration for purification and separation, titration for precise chemical analysis.
Definitions: What is Filtration?
Filtration is a physical separation process that removes solid particles from a liquid or gas by passing the mixture through a porous material or filter medium. This technique relies on the size difference between the particles and the filter pores to achieve separation, effectively isolating suspended solids from the fluid. Filtration is commonly used in laboratory, industrial, and environmental applications to purify liquids or gases and collect solid residues.
Definitions: What is Titration?
Titration is a quantitative chemical analysis method used to determine the concentration of an unknown solution by gradually adding a reagent of known concentration until a reaction completion point, called the equivalence point, is reached. This technique relies on indicators or pH meters to identify the endpoint, enabling precise calculation of the analyte's molarity. Unlike filtration, which separates solids from liquids physically, titration involves chemical reactions to measure substance concentrations in solutions.
Key Purposes and Applications
Filtration is primarily used to separate solid particles from liquids or gases, ensuring clarity in chemical mixtures and purification processes such as wastewater treatment and pharmaceutical manufacturing. Titration quantifies the concentration of an unknown solution by reacting it with a solution of known concentration, commonly applied in acid-base analysis, water quality testing, and pharmaceutical formulation. Both techniques are essential in laboratory analysis, with filtration focusing on physical separation and titration on quantitative chemical measurement.
Step-by-Step Process: Filtration
Filtration involves pouring a mixture through filter paper placed in a funnel to separate solid particles from liquids based on particle size. The solid residue collects on the filter paper while the filtrate passes through into a container below. Ensuring a steady flow and preventing clogging requires careful positioning of the funnel and timely replacement of filter paper during the process.
Step-by-Step Process: Titration
Titration involves precisely measuring a volume of titrant added to a solution of analyte until the reaction reaches the equivalence point, often indicated by a color change using an appropriate indicator. The step-by-step process starts with preparing a standard solution of known concentration, filling a burette with the titrant, and slowly adding it to the analyte solution in a conical flask while continuously swirling to mix. Recording the volume of titrant used at the endpoint allows calculation of the unknown concentration in the analyte through stoichiometric relationships.
Equipment and Materials Needed
Filtration requires equipment such as filter paper, a funnel, and a filtration apparatus like a Buchner funnel with a vacuum pump for solid-liquid separation, whereas titration involves a burette, pipette, conical flask, and appropriate indicators to measure the volume of a reagent added during a chemical reaction. Materials for filtration include the mixture to be separated and suitable filter media, while titration requires standard solutions and analytes prepared in precise concentrations. Proper equipment calibration and clean, compatible materials ensure accurate results in both techniques.
Advantages and Limitations of Filtration
Filtration offers the advantage of effectively separating solid particles from liquids or gases, providing a quick and simple method for purifying mixtures without chemical alterations. It is highly efficient for large-scale applications and is reusable with appropriate cleaning, but it is limited by its inability to separate dissolved substances and can be hindered by filter clogging or slow flow rates. Unlike titration, filtration does not quantify concentration but instead physically separates phases, making it unsuitable when chemical analysis precision is required.
Advantages and Limitations of Titration
Titration offers precise quantitative analysis with high accuracy, making it ideal for determining exact concentrations of solutions in chemical reactions. Its limitations include the requirement for skilled operators and the dependence on clear, distinct end-point indicators that can be subjective or affected by solution color. Unlike filtration, which separates solids from liquids, titration is less suited for mixtures involving insoluble impurities and does not physically remove substances but rather analyzes their concentration.
Filtration vs Titration: Choosing the Right Technique
Filtration and titration serve distinct purposes in chemical analysis, where filtration separates solids from liquids using a porous medium, ideal for isolating precipitates or clarifying solutions. Titration quantifies substance concentration through controlled reagent addition and endpoint detection, essential for precise chemical measurements. Choosing the right technique depends on whether physical separation or quantitative analysis is required in the experimental procedure.
Filtration Infographic
 libterm.com
libterm.com