Exothermic reactions release heat energy, making the surroundings warmer and causing a temperature increase. These processes are crucial in various applications, such as combustion engines, industrial manufacturing, and even biological metabolism. Discover how exothermic reactions impact your daily life and explore their practical uses in the rest of this article.
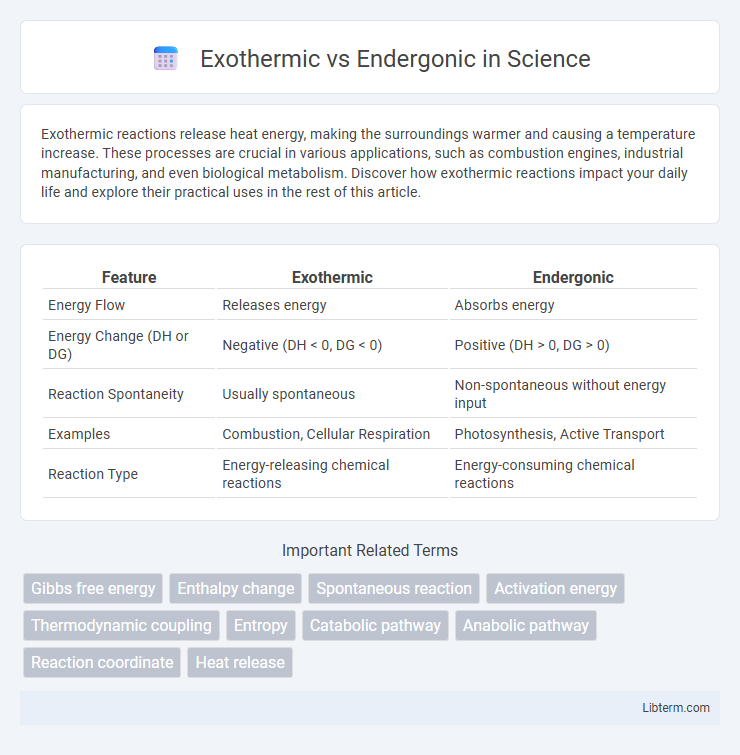
Table of Comparison
| Feature | Exothermic | Endergonic |
|---|---|---|
| Energy Flow | Releases energy | Absorbs energy |
| Energy Change (DH or DG) | Negative (DH < 0, DG < 0) | Positive (DH > 0, DG > 0) |
| Reaction Spontaneity | Usually spontaneous | Non-spontaneous without energy input |
| Examples | Combustion, Cellular Respiration | Photosynthesis, Active Transport |
| Reaction Type | Energy-releasing chemical reactions | Energy-consuming chemical reactions |
Introduction to Exothermic and Endergonic Reactions
Exothermic reactions release energy, often in the form of heat, as chemical bonds are broken and new bonds form with lower energy states. In contrast, endergonic reactions require an input of energy to proceed since they involve the absorption of energy to create higher-energy products. These fundamental differences determine reaction spontaneity and energy flow in chemical processes.
Defining Exothermic Reactions
Exothermic reactions release energy, usually in the form of heat, as the products have lower energy than the reactants, resulting in a negative enthalpy change (DH < 0). These reactions are characterized by the release of thermal energy to the surroundings, often observed in combustion and respiration processes. Understanding exothermic reactions is crucial for applications in chemical engineering, thermodynamics, and energy production.
Understanding Endergonic Reactions
Endergonic reactions require an input of energy to proceed, as they involve the absorption of free energy from their surroundings, resulting in a positive Gibbs free energy change (DG > 0). These reactions are non-spontaneous and often coupled with exergonic processes to drive metabolic pathways such as ATP synthesis and photosynthesis. Understanding the energy dynamics in endergonic reactions is crucial for studying cellular metabolism, biochemical pathways, and energy transfer mechanisms in living organisms.
Key Differences Between Exothermic and Endergonic Processes
Exothermic processes release energy, usually in the form of heat, resulting in a negative change in enthalpy (DH < 0), while endergonic processes require an input of energy to proceed, characterized by a positive Gibbs free energy change (DG > 0). Exothermic reactions often occur spontaneously as they increase the stability of the system, whereas endergonic reactions are non-spontaneous and need continuous energy supply to maintain progress. The key difference lies in energy flow direction: exothermic releases energy to the surroundings, endergonic absorbs energy from the surroundings.
Energy Flow in Chemical Reactions
Exothermic reactions release energy, usually in the form of heat, as reactants transform into products with lower energy, resulting in a negative Gibbs free energy change (DG < 0). Endergonic reactions absorb energy from the surroundings to convert reactants into higher-energy products, characterized by a positive Gibbs free energy change (DG > 0). The energy flow difference is crucial for understanding reaction spontaneity and is central to metabolic processes and chemical equilibrium.
Real-World Examples of Exothermic Reactions
Exothermic reactions release energy, often in the form of heat or light, making combustion of gasoline and respiration in living organisms prime examples. The rusting of iron is another common exothermic process where iron reacts with oxygen to form iron oxide, releasing heat. These reactions contrast with endergonic processes, which require energy input to proceed.
Common Applications of Endergonic Reactions
Endergonic reactions, which absorb energy from their surroundings, play a crucial role in biological processes such as photosynthesis, where light energy is converted into chemical energy stored in glucose molecules. These reactions are essential in cellular respiration pathways like ATP synthesis, enabling cells to store energy for various metabolic activities. Industrial applications include the production of synthetic compounds and pharmaceuticals, where energy input drives non-spontaneous chemical transformations.
Thermodynamic Principles: Enthalpy vs Free Energy
Exothermic reactions release enthalpy (DH < 0) by transferring heat to the surroundings, reflecting a decrease in system enthalpy. Endergonic reactions require an input of free energy (DG > 0), indicating a non-spontaneous process where the Gibbs free energy increases. Enthalpy changes govern heat exchange, while Gibbs free energy determines the spontaneity and direction of chemical reactions based on thermodynamic principles.
Importance in Biological Systems
Exothermic and endergonic reactions are crucial for maintaining energy balance in biological systems, with exothermic processes releasing energy essential for cellular activities and endergonic reactions requiring energy input to drive vital functions like biosynthesis. The coupling of these reactions enables organisms to efficiently convert nutrients into usable energy, supporting growth, reproduction, and homeostasis. Understanding this balance is fundamental for studying metabolism, enzyme function, and energy transfer within cells.
Summary: Choosing Between Exothermic and Endergonic Approaches
Exothermic reactions release energy, making them favorable for processes requiring heat output and spontaneity, whereas endergonic reactions absorb energy and are essential for energy-consuming metabolic pathways. Selecting between exothermic and endergonic approaches depends on the desired energy flow and thermodynamic feasibility within biological or chemical systems. Understanding the Gibbs free energy change (DG) guides the choice, with negative DG indicating exothermic spontaneity and positive DG indicating endergonic energy input.
Exothermic Infographic
 libterm.com
libterm.com