Positron emission is a type of radioactive decay where a proton inside an unstable nucleus is converted into a neutron, releasing a positron and a neutrino. This process plays a crucial role in medical imaging techniques like PET scans, which help detect metabolic activity in your body. Discover more about how positron emission impacts science and healthcare in the rest of the article.
Table of Comparison
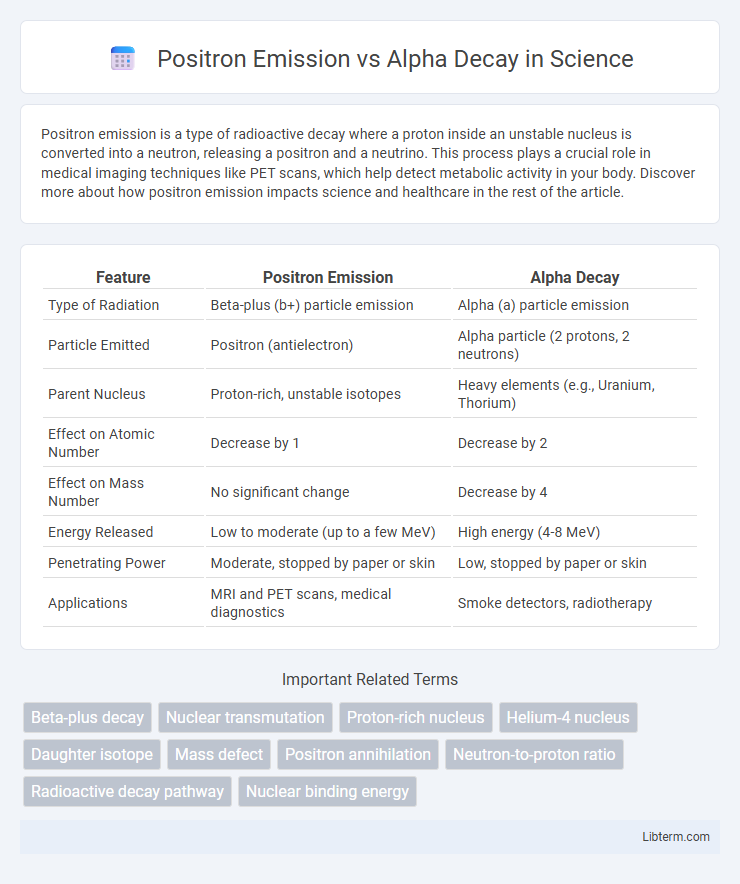
| Feature | Positron Emission | Alpha Decay |
|---|---|---|
| Type of Radiation | Beta-plus (b+) particle emission | Alpha (a) particle emission |
| Particle Emitted | Positron (antielectron) | Alpha particle (2 protons, 2 neutrons) |
| Parent Nucleus | Proton-rich, unstable isotopes | Heavy elements (e.g., Uranium, Thorium) |
| Effect on Atomic Number | Decrease by 1 | Decrease by 2 |
| Effect on Mass Number | No significant change | Decrease by 4 |
| Energy Released | Low to moderate (up to a few MeV) | High energy (4-8 MeV) |
| Penetrating Power | Moderate, stopped by paper or skin | Low, stopped by paper or skin |
| Applications | MRI and PET scans, medical diagnostics | Smoke detectors, radiotherapy |
Overview of Nuclear Decay Processes
Positron emission is a type of beta decay in which a proton inside the nucleus transforms into a neutron while releasing a positron and a neutrino, reducing the atomic number by one without changing the mass number. Alpha decay involves the emission of an alpha particle, consisting of two protons and two neutrons, which decreases the atomic number by two and the mass number by four, leading to a lighter nucleus. Both processes are forms of radioactive decay that allow unstable nuclei to reach more stable configurations through particle emission.
What is Positron Emission?
Positron emission is a type of radioactive decay in which a proton inside an unstable nucleus transforms into a neutron, releasing a positron and a neutrino. This process decreases the atomic number by one while the mass number remains unchanged, effectively converting the element into a different one. Positron emission is common in proton-rich nuclei and plays a crucial role in medical imaging techniques such as positron emission tomography (PET).
Understanding Alpha Decay
Alpha decay involves the emission of an alpha particle, consisting of two protons and two neutrons, from an unstable atomic nucleus, resulting in the reduction of the mass number by four and atomic number by two. This radioactive process is common in heavy elements like uranium and thorium, contributing to their natural radioactive series. Understanding alpha decay is crucial for applications in nuclear medicine, radiometric dating, and nuclear energy production.
Mechanisms Behind Positron Emission
Positron emission involves the conversion of a proton into a neutron within an unstable nucleus, releasing a positron and a neutrino; this process is governed by the weak nuclear force and reduces the atomic number by one while keeping the mass number constant. In contrast, alpha decay emits an alpha particle (two protons and two neutrons), significantly reducing both the atomic number and mass number of the parent nucleus. The mechanism behind positron emission is critical in medical imaging techniques like PET scans, where positrons annihilate with electrons, producing detectable gamma rays.
Mechanisms Behind Alpha Decay
Alpha decay involves the emission of an alpha particle, consisting of two protons and two neutrons, from an unstable nucleus to achieve greater stability. This process occurs when the nuclear force can no longer hold the tightly bound alpha particle within the nucleus, resulting in its ejection due to quantum tunneling through the nuclear potential barrier. In contrast, positron emission entails the conversion of a proton into a neutron and the subsequent release of a positron and a neutrino, fundamentally differing in particle type and nuclear transformation mechanisms.
Key Differences Between Positron Emission and Alpha Decay
Positron emission involves the release of a positron (a beta-plus particle) from a proton-rich nucleus, decreasing the atomic number by one while maintaining mass number. Alpha decay emits an alpha particle, consisting of two protons and two neutrons, resulting in a decrease of the atomic number by two and mass number by four. Positron emission primarily occurs in light nuclei undergoing proton-to-neutron conversion, whereas alpha decay typically happens in heavy nuclei to reduce instability.
Energy Changes in Positron Emission vs Alpha Decay
Positron emission involves the transformation of a proton into a neutron within the nucleus, releasing a positron and a neutrino, resulting in a decrease in atomic number by one while conserving mass number, with an energy change typically in the range of several hundred keV to a few MeV due to mass-energy equivalence. Alpha decay emits an alpha particle (two protons and two neutrons), leading to a more substantial drop in both atomic and mass numbers, accompanied by a release of significantly higher energy, often between 4 to 9 MeV, attributed to the strong nuclear binding energy difference. The energy released in alpha decay is generally greater than in positron emission because alpha particles carry away more kinetic energy and the daughter nucleus achieves a more stable configuration.
Effects on Atomic Nucleus
Positron emission transforms a proton into a neutron within the atomic nucleus, decreasing the atomic number by one while keeping the mass number constant, effectively converting the element into its neighboring lower atomic number isotope. Alpha decay involves the emission of an alpha particle, composed of two protons and two neutrons, resulting in a reduction of both the atomic number by two and the mass number by four, significantly altering the nucleus's size and composition. These nuclear transformations directly impact the stability and radioactive behavior of the parent atom, influencing subsequent decay pathways and element transmutation.
Real-World Applications and Examples
Positron emission is crucial in medical imaging techniques such as Positron Emission Tomography (PET) scans, allowing detailed visualization of metabolic processes and cancer detection. Alpha decay plays a significant role in radiometric dating, enabling the age determination of rocks and fossils through measurement of emitted alpha particles. Both processes contribute to nuclear medicine, environmental science, and geological research by providing precise data on atomic and molecular interactions.
Summary: Comparing Positron Emission and Alpha Decay
Positron emission involves the release of a positron and a neutrino from a proton-rich nucleus, converting a proton into a neutron and decreasing the atomic number by one without changing the mass number. Alpha decay emits a helium-4 nucleus (two protons and two neutrons), significantly reducing both the atomic number by two and the mass number by four. These nuclear decay processes differ in particle emission, changes in atomic composition, and occur in distinct isotopic environments.
Positron Emission Infographic
 libterm.com
libterm.com