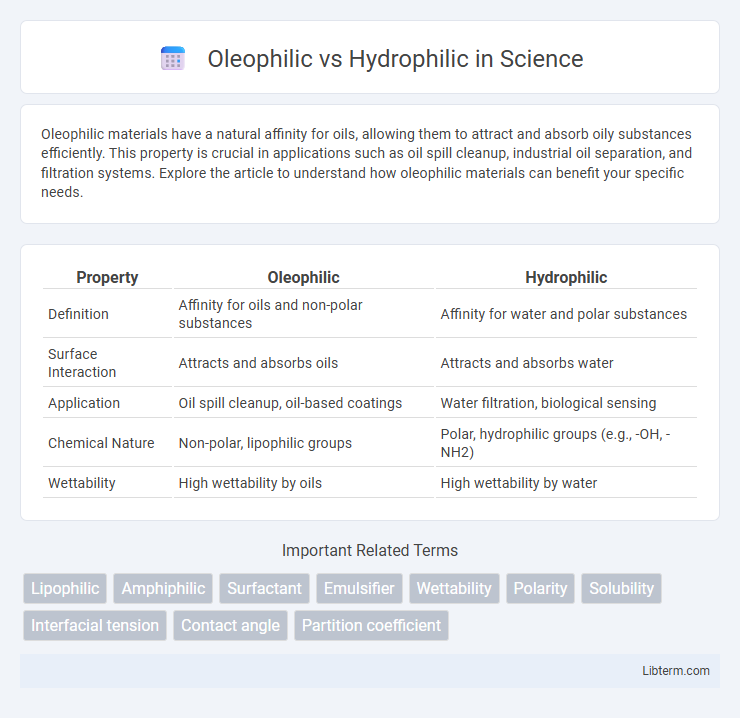
Oleophilic materials have a natural affinity for oils, allowing them to attract and absorb oily substances efficiently. This property is crucial in applications such as oil spill cleanup, industrial oil separation, and filtration systems. Explore the article to understand how oleophilic materials can benefit your specific needs.
Table of Comparison
| Property | Oleophilic | Hydrophilic |
|---|---|---|
| Definition | Affinity for oils and non-polar substances | Affinity for water and polar substances |
| Surface Interaction | Attracts and absorbs oils | Attracts and absorbs water |
| Application | Oil spill cleanup, oil-based coatings | Water filtration, biological sensing |
| Chemical Nature | Non-polar, lipophilic groups | Polar, hydrophilic groups (e.g., -OH, -NH2) |
| Wettability | High wettability by oils | High wettability by water |
Introduction to Oleophilic and Hydrophilic Properties
Oleophilic materials exhibit a strong affinity for oils and non-polar substances, making them ideal for applications involving oil absorption or separation. Hydrophilic materials attract water molecules due to their polar nature, enhancing their effectiveness in water filtration, coating, and moisture retention. Understanding the contrast between oleophilic and hydrophilic properties is crucial for designing advanced materials in environmental cleanup, textiles, and chemical processing.
Defining Oleophilic: Characteristics and Examples
Oleophilic materials exhibit a strong affinity for oils and non-polar substances, efficiently attracting and absorbing hydrophobic compounds. Common examples include silicone-based compounds and certain polymers like polyethylene, which are widely used in oil spill cleanup and industrial applications. These materials' oleophilic properties make them essential for separating oil from water, enhancing environmental remediation processes.
What Does Hydrophilic Mean? Key Features Explained
Hydrophilic refers to substances or molecules that have an affinity for water, readily absorbing or dissolving in it due to their polar nature. Key features of hydrophilic materials include strong intermolecular forces with water, such as hydrogen bonding, and high solubility in aqueous environments. Common examples include salts, sugars, and certain polymers like polyethylene glycol, which are widely utilized in applications requiring water interaction or moisture retention.
Molecular Structure Differences: Oleophilic vs Hydrophilic
Oleophilic molecules possess nonpolar hydrocarbon chains that enable strong interactions with oils and other lipophilic substances through van der Waals forces, whereas hydrophilic molecules contain polar functional groups such as hydroxyl, carboxyl, or amine groups that form hydrogen bonds with water molecules. The molecular structure of oleophilic compounds typically lacks polarity, favoring solubility in nonpolar solvents, while hydrophilic molecules exhibit significant polarity, enhancing solubility in aqueous environments. These distinct molecular characteristics determine their affinity and solubility preferences, influencing applications in fields like drug delivery, coatings, and surfactant design.
Role in Chemistry and Material Science
Oleophilic materials exhibit strong affinity for oils and nonpolar substances, making them essential in applications such as oil spill cleanup and lubrication. Hydrophilic substances attract water molecules due to their polar nature, playing a crucial role in adsorption, catalysis, and biomedical devices. Understanding the oleophilic-hydrophilic balance allows scientists to design surface coatings and membranes with tailored wettability for advanced chemical separations and material performance.
Oleophilic and Hydrophilic Applications in Everyday Life
Oleophilic materials attract and absorb oils, making them essential in applications such as oil spill cleanup, grease filtration, and oil-based cosmetics. Hydrophilic substances readily absorb water, finding common use in contact lenses, wound dressings, and moisture-wicking fabrics. Both properties enhance the effectiveness of products designed to manage either oil or water, improving performance and functionality in daily use.
Surface Interactions: How Materials Behave with Water and Oils
Oleophilic materials exhibit strong affinity for oils, allowing them to easily absorb or attract oily substances, while hydrophilic materials preferentially interact with water, promoting wetting and adhesion. Surface energy plays a crucial role, where oleophilic surfaces have low surface energy favoring nonpolar oil molecules, and hydrophilic surfaces possess high surface energy, attracting polar water molecules. These differences impact applications in coatings, filtration, and chemical separation by determining whether a material repels or absorbs water or oil-based contaminants.
Impact on Product Formulation: Cosmetics, Pharmaceuticals, and Cleaning Agents
Oleophilic substances attract oils and fats, enhancing the solubility and stability of lipophilic active ingredients in cosmetics and pharmaceuticals, crucial for effective delivery and absorption. Hydrophilic materials, which readily interact with water, improve the dispersion and bioavailability of hydrophilic compounds, playing a vital role in emulsions and aqueous cleaning agents. Optimizing the balance between oleophilic and hydrophilic properties ensures efficient formulation performance, stability, and user experience across diverse product applications.
Testing and Identifying Oleophilic and Hydrophilic Substances
Testing and identifying oleophilic and hydrophilic substances primarily involve contact angle measurements and absorption tests to determine affinity for oil or water. Oleophilic materials exhibit low contact angles with oils, indicating strong attraction, whereas hydrophilic substances show low contact angles with water, reflecting their affinity towards moisture. Spectroscopic methods and chromatography can further analyze surface chemistry to confirm the oleophilic or hydrophilic nature based on molecular interactions.
Future Trends and Innovations in Oleophilic and Hydrophilic Materials
Future trends in oleophilic and hydrophilic materials emphasize the development of advanced coatings and membranes for environmental remediation, such as oil spill cleanup and water purification. Innovations in nanotechnology enable the creation of superoleophilic and superhydrophilic surfaces that enhance selectivity and efficiency in separation processes. Researchers are also exploring bio-inspired materials that mimic natural surfaces to achieve sustainable and energy-efficient solutions for industrial applications.
Oleophilic Infographic
 libterm.com
libterm.com