Proteolysis is the biological process where proteins are broken down into smaller peptides or amino acids by enzymes called proteases, playing a crucial role in cellular function and metabolism. This mechanism is essential for regulating protein activity, recycling cellular components, and maintaining homeostasis. Discover how proteolysis impacts your body and its vital importance by reading the rest of the article.
Table of Comparison
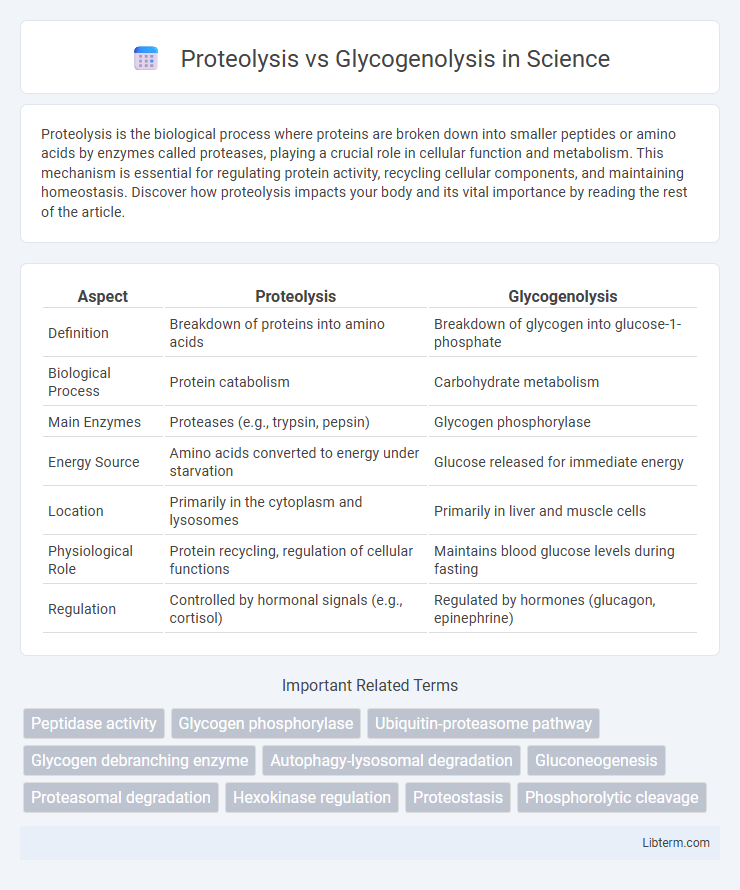
| Aspect | Proteolysis | Glycogenolysis |
|---|---|---|
| Definition | Breakdown of proteins into amino acids | Breakdown of glycogen into glucose-1-phosphate |
| Biological Process | Protein catabolism | Carbohydrate metabolism |
| Main Enzymes | Proteases (e.g., trypsin, pepsin) | Glycogen phosphorylase |
| Energy Source | Amino acids converted to energy under starvation | Glucose released for immediate energy |
| Location | Primarily in the cytoplasm and lysosomes | Primarily in liver and muscle cells |
| Physiological Role | Protein recycling, regulation of cellular functions | Maintains blood glucose levels during fasting |
| Regulation | Controlled by hormonal signals (e.g., cortisol) | Regulated by hormones (glucagon, epinephrine) |
Introduction to Proteolysis and Glycogenolysis
Proteolysis is the biochemical process involving the breakdown of proteins into smaller peptides or amino acids through enzymatic action, playing a crucial role in cellular metabolism and protein turnover. Glycogenolysis refers to the metabolic pathway where glycogen, the storage form of glucose in liver and muscle tissues, is enzymatically degraded to release glucose-1-phosphate, supplying energy during periods of fasting or intense exercise. Both processes are essential catabolic mechanisms that regulate energy availability and maintain metabolic homeostasis in response to physiological demands.
Definitions and Key Differences
Proteolysis is the biochemical process of breaking down proteins into smaller peptides or amino acids through enzymatic action, essential for protein turnover and cellular regulation. Glycogenolysis involves the enzymatic breakdown of glycogen into glucose-1-phosphate to maintain blood glucose levels during fasting or increased energy demands. The key difference lies in substrates and products: proteolysis targets proteins yielding amino acids, while glycogenolysis degrades glycogen to release glucose for energy metabolism.
Biological Functions of Proteolysis
Proteolysis is the biological process that breaks down proteins into amino acids, essential for cellular repair, enzyme activation, and regulation of metabolic pathways. This catabolic function supports tissue remodeling, immune responses, and the removal of damaged or misfolded proteins to maintain cellular homeostasis. In contrast, glycogenolysis specifically mobilizes glucose from glycogen stores to provide immediate energy during fasting or strenuous activity.
Biological Functions of Glycogenolysis
Glycogenolysis plays a crucial role in maintaining blood glucose levels by breaking down glycogen stored in liver and muscle cells into glucose-1-phosphate, which is then converted to glucose-6-phosphate for energy production or released into the bloodstream. This catabolic process provides a rapid source of glucose during fasting, exercise, or stress, supporting cellular respiration and ATP synthesis. Unlike proteolysis, which degrades proteins into amino acids primarily for tissue remodeling and energy under prolonged starvation, glycogenolysis specifically targets glycogen to ensure immediate glucose availability and metabolic homeostasis.
Enzymes Involved in Proteolysis
Proteolysis involves enzymes such as pepsin, trypsin, chymotrypsin, and proteases like cathepsins, which break down proteins into peptides and amino acids. In contrast, glycogenolysis primarily relies on glycogen phosphorylase to degrade glycogen into glucose-1-phosphate. The distinct enzymatic pathways highlight proteolysis's role in protein catabolism versus glycogenolysis's function in carbohydrate metabolism.
Enzymes Involved in Glycogenolysis
Glycogenolysis involves key enzymes such as glycogen phosphorylase, which catalyzes the cleavage of glucose units from glycogen by breaking a-1,4 glycosidic bonds, and debranching enzyme, which remodels glycogen by transferring and hydrolyzing a-1,6 linkages. In contrast, proteolysis primarily utilizes proteases like pepsin, trypsin, and chymotrypsin to hydrolyze peptide bonds in proteins into amino acids. The enzymatic specificity in glycogenolysis ensures the rapid mobilization of glucose-1-phosphate, critical for maintaining blood glucose levels during fasting or intense exercise.
Regulatory Mechanisms
Proteolysis is regulated primarily by hormonal signals such as cortisol and insulin, which modulate protease activity and protein degradation rates in tissues. Glycogenolysis is controlled by key enzymes like glycogen phosphorylase, activated through hormonal pathways involving glucagon and epinephrine, which trigger cyclic AMP (cAMP) signaling cascades. Both processes rely on intricate feedback mechanisms to balance energy needs and maintain metabolic homeostasis.
Physiological Roles and Importance
Proteolysis breaks down proteins into amino acids, supplying substrates for gluconeogenesis and supporting muscle repair and immune function, crucial during fasting and stress. Glycogenolysis rapidly mobilizes stored glycogen into glucose, maintaining blood glucose levels and providing immediate energy during intense physical activity or between meals. Both processes are vital metabolic pathways ensuring energy homeostasis and cellular function under varying physiological conditions.
Clinical Relevance and Disorders
Proteolysis, the breakdown of proteins into amino acids, plays a crucial role in muscle wasting diseases such as sarcopenia and cachexia, where excessive protein degradation leads to loss of muscle mass and strength. Glycogenolysis, the enzymatic breakdown of glycogen to glucose, is vital in maintaining blood glucose levels; defects in this pathway cause glycogen storage diseases like Von Gierke's disease and McArdle's disease, leading to hypoglycemia and muscle fatigue. Both processes are key therapeutic targets in metabolic disorders, with proteolysis inhibitors and glycogen phosphorylase modulators under investigation for clinical management.
Summary and Key Takeaways
Proteolysis is the biochemical process that breaks down proteins into amino acids, serving as a critical mechanism for cellular repair, energy production, and regulation of metabolic pathways. Glycogenolysis involves the enzymatic breakdown of glycogen into glucose-1-phosphate and glucose molecules, providing a rapid source of energy during periods of fasting or increased muscular activity. Key distinctions include substrate type, enzymatic pathways, and metabolic roles, with proteolysis focusing on protein catabolism and glycogenolysis on carbohydrate mobilization for immediate energy needs.
Proteolysis Infographic
 libterm.com
libterm.com