Lipophilic substances have an affinity for lipids or fats, enabling them to easily dissolve in non-polar solvents such as oils and fats, which influences their behavior in biological systems and industrial applications. Understanding the lipophilic nature of molecules is crucial for fields like pharmacology, where drug solubility affects absorption, distribution, and efficacy within the body. Explore the rest of this article to uncover how lipophilicity impacts your health and various scientific advancements.
Table of Comparison
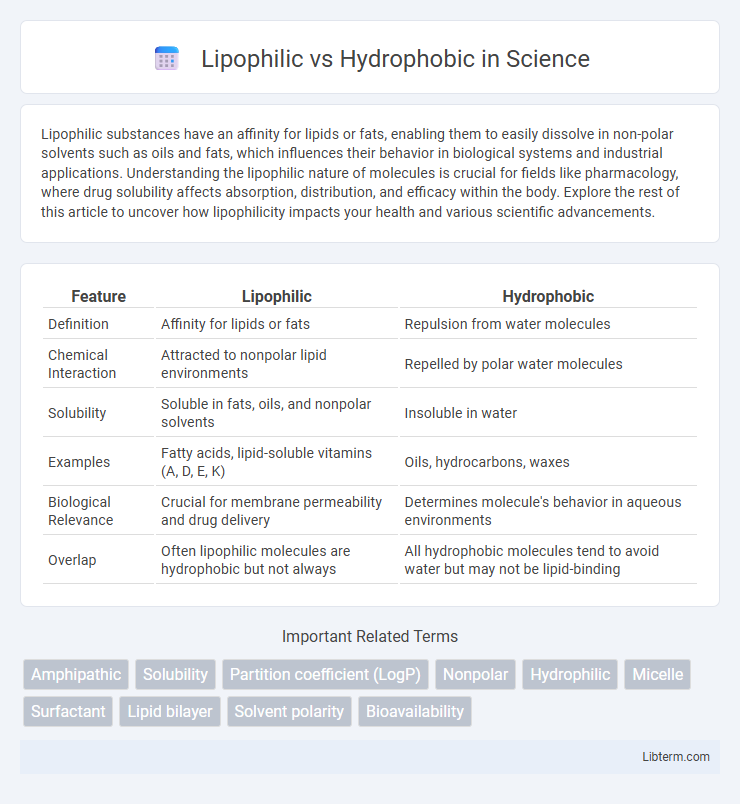
| Feature | Lipophilic | Hydrophobic |
|---|---|---|
| Definition | Affinity for lipids or fats | Repulsion from water molecules |
| Chemical Interaction | Attracted to nonpolar lipid environments | Repelled by polar water molecules |
| Solubility | Soluble in fats, oils, and nonpolar solvents | Insoluble in water |
| Examples | Fatty acids, lipid-soluble vitamins (A, D, E, K) | Oils, hydrocarbons, waxes |
| Biological Relevance | Crucial for membrane permeability and drug delivery | Determines molecule's behavior in aqueous environments |
| Overlap | Often lipophilic molecules are hydrophobic but not always | All hydrophobic molecules tend to avoid water but may not be lipid-binding |
Understanding Lipophilic and Hydrophobic Properties
Lipophilic substances exhibit an affinity for lipids and dissolve readily in nonpolar solvents, whereas hydrophobic molecules repel water and avoid interaction with polar solvents. Both terms describe nonpolar characteristics but differ in context: lipophilic emphasizes affinity toward fats or oils, while hydrophobic focuses on water resistance. Understanding these properties is crucial for drug design, as lipophilicity influences membrane permeability and hydrophobicity affects solubility and molecular interactions.
Definition of Lipophilic Compounds
Lipophilic compounds are chemical substances that exhibit a strong affinity for lipid environments due to their nonpolar molecular structure, allowing them to dissolve readily in fats, oils, and other hydrophobic solvents. These compounds typically possess long hydrocarbon chains or aromatic rings that enhance their solubility in lipid membranes and organic solvents, distinguishing them from hydrophilic or water-attracting molecules. Lipophilicity plays a crucial role in drug design, environmental science, and biochemistry by influencing absorption, distribution, and interaction within biological systems.
Definition of Hydrophobic Compounds
Hydrophobic compounds are molecules that repel water and do not readily dissolve in aqueous environments due to their nonpolar nature. These compounds tend to aggregate together, minimizing their exposure to water, which is a polar solvent. Unlike lipophilic substances that specifically dissolve in lipids or fats, hydrophobic compounds avoid interactions with water without necessarily having an affinity for oils.
Chemical Structure Differences
Lipophilic molecules possess nonpolar chemical structures with long hydrocarbon chains or rings that readily dissolve in fats and oils, whereas hydrophobic molecules exhibit a more general aversion to water, often due to nonpolar characteristics but can also include certain polar groups arranged to minimize water interaction. Lipophilicity specifically refers to affinity for lipid environments linked to molecular polarity and electron distribution, influencing solubility and membrane permeability. The key structural difference lies in lipophilic compounds' tailored hydrocarbon frameworks that promote lipid solubility, contrasting with hydrophobic molecules which are defined broadly by their incapacity to interact favorably with aqueous surroundings.
Interaction with Water Molecules
Lipophilic molecules exhibit an affinity for lipid environments, often dissolving readily in nonpolar solvents while displaying limited interaction with water molecules due to their preference for hydrophobic interactions. Hydrophobic molecules repel water, causing water molecules to form structured hydrogen bond networks around them, minimizing contact and leading to phenomena such as micelle formation or phase separation in aqueous solutions. The distinction lies in lipophilic substances' ability to integrate into lipid bilayers, whereas hydrophobic substances primarily avoid aqueous environments by aggregating or clustering together.
Biological Significance of Lipophilicity
Lipophilicity plays a critical role in drug design by influencing membrane permeability and bioavailability, as lipophilic molecules can easily traverse lipid bilayers of cell membranes. Unlike hydrophobicity, which denotes water avoidance, lipophilicity specifically relates to affinity for lipid environments, affecting a compound's distribution within biological systems. This property determines the absorption, distribution, metabolism, and excretion (ADME) of pharmaceuticals, thereby crucially impacting therapeutic efficacy and toxicity profiles.
Biological Significance of Hydrophobicity
Hydrophobicity plays a crucial role in biological systems by driving the folding of proteins and the formation of cell membranes, where hydrophobic amino acid residues cluster away from aqueous environments, stabilizing three-dimensional structures. Lipophilic molecules, while also nonpolar, specifically interact with lipid environments, influencing membrane permeability and signaling processes. The distinction between hydrophobic and lipophilic properties is essential for understanding molecular behavior in biological contexts such as drug design and membrane dynamics.
Applications in Drug Design
Lipophilic drug molecules readily cross cell membranes, enhancing absorption and bioavailability, while hydrophobic characteristics influence drug solubility and distribution in biological systems. Optimizing lipophilicity is crucial for targeting intracellular receptors and improving membrane permeability, whereas balancing hydrophobicity reduces non-specific binding and toxicity. Structure-activity relationship (SAR) studies frequently adjust these properties to achieve optimal pharmacokinetics and therapeutic efficacy.
Common Misconceptions: Lipophilic vs Hydrophobic
Lipophilic and hydrophobic are often incorrectly used interchangeably despite their distinct chemical properties. Lipophilic substances have an affinity for lipids and dissolve well in fats and oils, whereas hydrophobic substances repel water but may not necessarily dissolve in lipids. Understanding this difference is crucial for applications in pharmaceuticals, biochemistry, and material science where molecular interactions with water and lipids are involved.
Summary: Key Differences and Practical Implications
Lipophilic substances exhibit an affinity for lipids and dissolve readily in fats, whereas hydrophobic substances repel water and do not mix with it. Lipophilicity is crucial in drug design for targeting cell membranes, while hydrophobicity influences molecular interactions in aqueous environments. Understanding these differences aids in predicting compound behavior in biological systems and environmental contexts.
Lipophilic Infographic
 libterm.com
libterm.com