Noncompetitive inhibition occurs when an inhibitor binds to an enzyme at a site other than the active site, altering the enzyme's shape and reducing its activity regardless of substrate concentration. This type of inhibition cannot be overcome by simply increasing substrate levels, making it a critical mechanism in regulating metabolic pathways. Explore the full article to understand how noncompetitive inhibitors impact enzyme function and their significance in drug design.
Table of Comparison
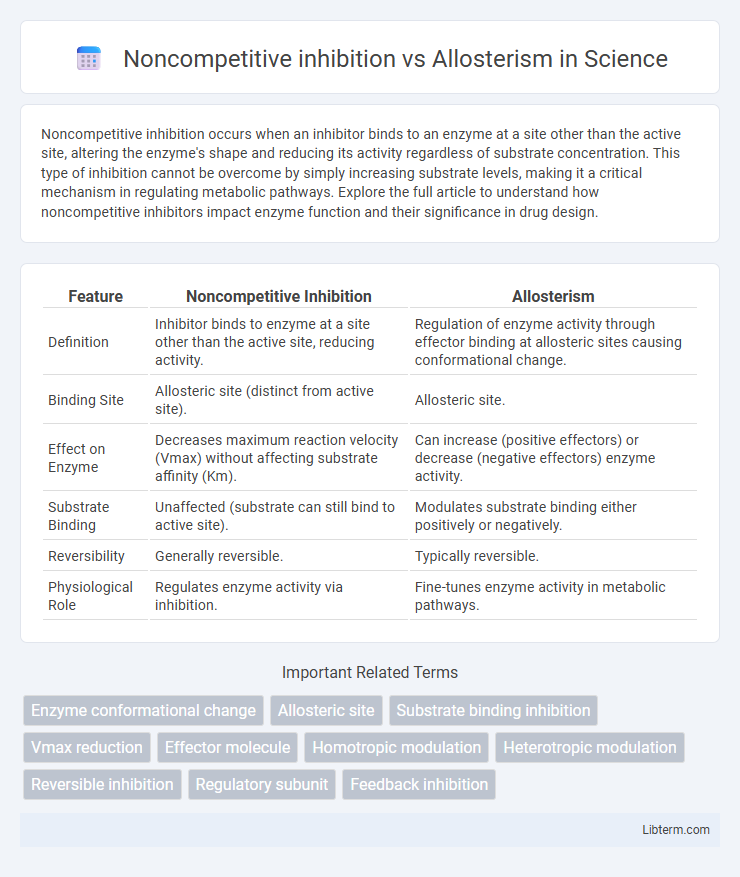
| Feature | Noncompetitive Inhibition | Allosterism |
|---|---|---|
| Definition | Inhibitor binds to enzyme at a site other than the active site, reducing activity. | Regulation of enzyme activity through effector binding at allosteric sites causing conformational change. |
| Binding Site | Allosteric site (distinct from active site). | Allosteric site. |
| Effect on Enzyme | Decreases maximum reaction velocity (Vmax) without affecting substrate affinity (Km). | Can increase (positive effectors) or decrease (negative effectors) enzyme activity. |
| Substrate Binding | Unaffected (substrate can still bind to active site). | Modulates substrate binding either positively or negatively. |
| Reversibility | Generally reversible. | Typically reversible. |
| Physiological Role | Regulates enzyme activity via inhibition. | Fine-tunes enzyme activity in metabolic pathways. |
Introduction to Enzyme Regulation
Noncompetitive inhibition occurs when an inhibitor binds to an enzyme at a site distinct from the active site, altering enzyme activity without affecting substrate binding. Allosterism involves the regulation of enzyme activity through the binding of effectors at allosteric sites, which induces conformational changes that modulate catalytic function. Both mechanisms are crucial in enzyme regulation, enabling precise control of metabolic pathways by modulating enzyme activity beyond substrate availability.
Defining Noncompetitive Inhibition
Noncompetitive inhibition occurs when an inhibitor binds to an enzyme at a site other than the active site, causing a change in enzyme conformation that reduces its activity regardless of substrate concentration. Unlike competitive inhibition, the inhibitor does not directly compete with the substrate for binding but decreases the maximum reaction velocity (Vmax) without affecting the substrate affinity (Km). Allosterism encompasses a broader mechanism where regulatory molecules bind allosteric sites, modulating enzyme activity either positively or negatively, with noncompetitive inhibition representing a specific type of negative allosteric regulation.
Understanding Allosterism in Enzymes
Allosterism in enzymes involves the binding of effectors at sites distinct from the active site, inducing conformational changes that modulate enzymatic activity. Noncompetitive inhibition occurs when inhibitors bind to an enzyme at allosteric sites, reducing its activity regardless of substrate concentration. Understanding allosterism reveals how enzymes regulate metabolic pathways through dynamic conformational shifts that fine-tune catalytic efficiency and responsiveness.
Key Mechanistic Differences
Noncompetitive inhibition occurs when an inhibitor binds to an enzyme at a site other than the active site, reducing enzyme activity regardless of substrate concentration. Allosterism involves the binding of effectors to specific regulatory sites, causing conformational changes that modulate enzyme activity positively or negatively. The key mechanistic difference lies in noncompetitive inhibition typically leading to decreased maximum velocity without affecting substrate affinity, whereas allosterism can either enhance or inhibit enzyme function by altering both affinity and catalytic activity through conformational shifts.
Binding Sites: Active vs Allosteric
Noncompetitive inhibition occurs when an inhibitor binds to an enzyme's allosteric site, causing a conformational change that reduces enzymatic activity without directly blocking the active site. Allosterism involves the binding of effectors to sites distinct from the active site, modulating enzyme function either positively or negatively through structural alterations. Both mechanisms rely on allosteric binding sites, but noncompetitive inhibition specifically decreases catalytic efficiency, whereas allosterism can fine-tune enzymatic activity in multiple ways.
Effects on Enzyme Kinetics
Noncompetitive inhibition decreases the maximum reaction velocity (Vmax) without affecting the substrate affinity (Km), as the inhibitor binds to an allosteric site distinct from the active site. Allosterism modulates enzyme activity by inducing conformational changes that can either increase or decrease both Vmax and Km, depending on whether the effector is an activator or inhibitor. Noncompetitive inhibitors reduce catalytic efficiency irrespective of substrate concentration, while allosteric regulation provides dynamic control over enzyme kinetics through reversible structural alterations.
Examples in Biological Systems
Noncompetitive inhibition occurs when an inhibitor binds to an enzyme at a site other than the active site, exemplified by the inhibition of cytochrome P450 enzymes by drugs like ketoconazole. Allosterism involves the binding of effectors at specific regulatory sites, demonstrated by hemoglobin's oxygen-binding, where 2,3-bisphosphoglycerate (2,3-BPG) modulates oxygen affinity. Both mechanisms are crucial for cellular regulation, with noncompetitive inhibitors often acting irreversibly or reversibly to reduce enzyme activity, while allosteric effectors fine-tune protein function through conformational changes.
Physiological Significance
Noncompetitive inhibition and allosterism both regulate enzyme activity by binding to sites distinct from the active site, influencing physiological processes such as metabolism and signal transduction. Noncompetitive inhibitors reduce enzyme activity regardless of substrate concentration, crucial for controlling pathways where substrate fluctuations occur, while allosteric modulation fine-tunes enzyme responsiveness, enabling dynamic adaptation to cellular conditions. These mechanisms ensure precise regulation of biological functions, maintaining homeostasis and facilitating cellular responses to environmental changes.
Experimental Identification Methods
Noncompetitive inhibition is experimentally identified through enzyme kinetics by observing unchanged Km values and decreased Vmax in Lineweaver-Burk plots, indicating inhibitor binding to an allosteric site without affecting substrate affinity. Allosterism detection involves measuring changes in enzyme activity or conformational structure upon effector binding, often using techniques like fluorescence spectroscopy, isothermal titration calorimetry (ITC), or X-ray crystallography to observe conformational shifts. Distinguishing noncompetitive inhibition from allosterism requires combining kinetic assays with structural or binding studies to confirm the inhibitor's effect on enzyme functionality and allosteric site involvement.
Therapeutic and Industrial Implications
Noncompetitive inhibition directly reduces enzyme activity by binding to an enzyme's allosteric site, providing therapeutic advantages in drug design by selectively modulating enzyme function without competing with substrate concentration. Allosterism, involving conformational changes induced by effector molecules, is crucial for industrial biocatalysis as it allows fine-tuning of enzyme activity and stability under varying conditions. Both mechanisms enable targeted regulation of biochemical pathways, enhancing drug efficacy and optimizing industrial enzyme processes.
Noncompetitive inhibition Infographic
 libterm.com
libterm.com