Chemiluminescence is the emission of light resulting from a chemical reaction without the involvement of heat. This process is widely used in forensic science, medical diagnostics, and environmental monitoring for its sensitive detection capabilities. Discover how chemiluminescence can enhance your analytical techniques by exploring the details in the article.
Table of Comparison
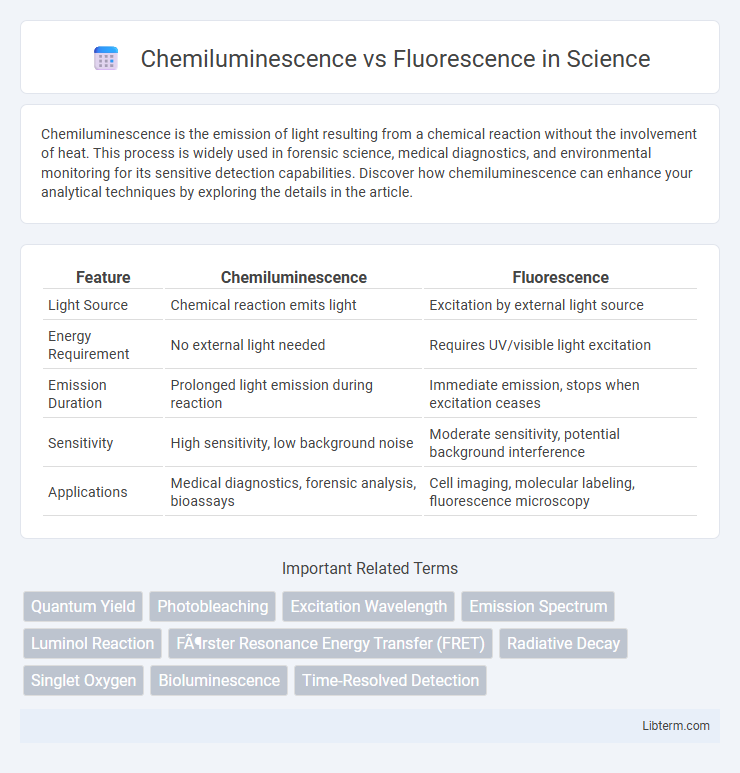
| Feature | Chemiluminescence | Fluorescence |
|---|---|---|
| Light Source | Chemical reaction emits light | Excitation by external light source |
| Energy Requirement | No external light needed | Requires UV/visible light excitation |
| Emission Duration | Prolonged light emission during reaction | Immediate emission, stops when excitation ceases |
| Sensitivity | High sensitivity, low background noise | Moderate sensitivity, potential background interference |
| Applications | Medical diagnostics, forensic analysis, bioassays | Cell imaging, molecular labeling, fluorescence microscopy |
Introduction to Chemiluminescence and Fluorescence
Chemiluminescence is the emission of light resulting from a chemical reaction without requiring an external light source, commonly used in bioassays and analytical chemistry for sensitive detection. Fluorescence occurs when a substance absorbs light at a specific wavelength and rapidly re-emits it at a longer wavelength, utilized extensively in microscopy, sensing, and imaging. Both phenomena are critical in scientific research for detecting and visualizing molecules, with chemiluminescence relying on chemical energy and fluorescence on photonic excitation.
Fundamental Principles of Chemiluminescence
Chemiluminescence is a chemical process in which light is emitted as a result of a spontaneous chemical reaction, differing fundamentally from fluorescence, where light is emitted after excitation by an external light source. The energy released during the chemical reaction excites molecules to a higher electronic state, and light is emitted as these molecules return to their ground state without the need for photon absorption. This intrinsic property of chemiluminescence enables sensitive detection in analytical applications, such as immunoassays and biosensors, by directly converting chemical energy into visible light.
Core Mechanisms of Fluorescence
Fluorescence occurs when a molecule absorbs photons, exciting electrons to a higher energy state, and then quickly returns to the ground state by emitting light at a longer wavelength. Unlike chemiluminescence, which produces light through a chemical reaction without external light excitation, fluorescence requires an external light source for excitation. The core mechanism involves absorption, non-radiative relaxation, and radiative emission, typically within nanoseconds after photon absorption.
Key Differences Between Chemiluminescence and Fluorescence
Chemiluminescence generates light through a chemical reaction without external light excitation, while fluorescence requires absorption of light to emit photons. Chemiluminescence typically produces a longer-lasting, steady light emission, whereas fluorescence involves rapid emission with a short lifetime following excitation. The mechanisms differ fundamentally: chemiluminescence relies on chemical energy conversion, and fluorescence depends on electronic excitation and relaxation.
Sensitivity and Detection Limits
Chemiluminescence exhibits higher sensitivity and lower detection limits compared to fluorescence, due to its direct light emission from a chemical reaction without the need for external excitation. Fluorescence often suffers from background noise and photobleaching, limiting its sensitivity in trace detection scenarios. Chemiluminescent assays can detect analytes at femtomolar to attomolar concentrations, making them more suitable for applications requiring ultra-trace analysis.
Instrumentation and Technical Requirements
Chemiluminescence requires sensitive photodetectors such as photomultiplier tubes (PMTs) and does not need an external light source, enhancing signal-to-noise ratio and reducing background interference during measurement. Fluorescence instrumentation depends on excitation sources like xenon lamps or lasers and employs filters or monochromators to isolate emission wavelengths, demanding precise optical components and stable light intensity. Both techniques benefit from temperature control and dark environment enclosures to optimize detection sensitivity and accuracy in analytical applications.
Common Applications in Research and Industry
Chemiluminescence is widely used in biomedical assays and environmental monitoring due to its high sensitivity and low background noise, enabling precise detection of trace substances. Fluorescence finds extensive applications in molecular biology, medical diagnostics, and materials science thanks to its ability to provide detailed spatial imaging and quantitative analysis of biomolecules. Both techniques are integral in pharmaceutical development and forensic analysis, with chemiluminescence favored for its signal clarity and fluorescence for imaging capabilities.
Advantages and Limitations of Chemiluminescence
Chemiluminescence offers high sensitivity and low background noise due to the absence of external light sources, making it advantageous for detecting low-abundance biomolecules. Its limitation includes the transient nature of the emitted light, requiring precise timing and specialized equipment for accurate measurement. Unlike fluorescence, chemiluminescence does not suffer from photobleaching but may have limited multiplexing capabilities due to narrower emission spectra.
Pros and Cons of Fluorescence Techniques
Fluorescence techniques offer high sensitivity and specificity, enabling the detection of low-abundance molecules with precise spatial resolution in biological samples. They allow real-time imaging and multiplexing capabilities, but suffer from photobleaching, limited penetration depth, and potential background autofluorescence interference. Despite these challenges, fluorescence remains a dominant method for molecular labeling, quantitative analysis, and diagnostic applications due to its versatility and well-developed instrumentation.
Future Trends in Luminescent Detection Methods
Future trends in luminescent detection methods emphasize enhanced sensitivity and specificity through novel chemiluminescence and fluorescence techniques. Innovations include the development of nanomaterial-based probes and advanced quantum dot fluorophores that enable real-time, multiplexed bioassays with ultra-low detection limits. Integration with microfluidic platforms and machine learning algorithms aims to revolutionize portable diagnostics and environmental monitoring applications.
Chemiluminescence Infographic
 libterm.com
libterm.com