Irreversible processes are fundamental in thermodynamics, representing changes that cannot spontaneously return to their original state. Understanding these processes unveils critical insights into energy efficiency and entropy increase. Explore the rest of the article to discover how irreversible phenomena impact your daily life and technological advancements.
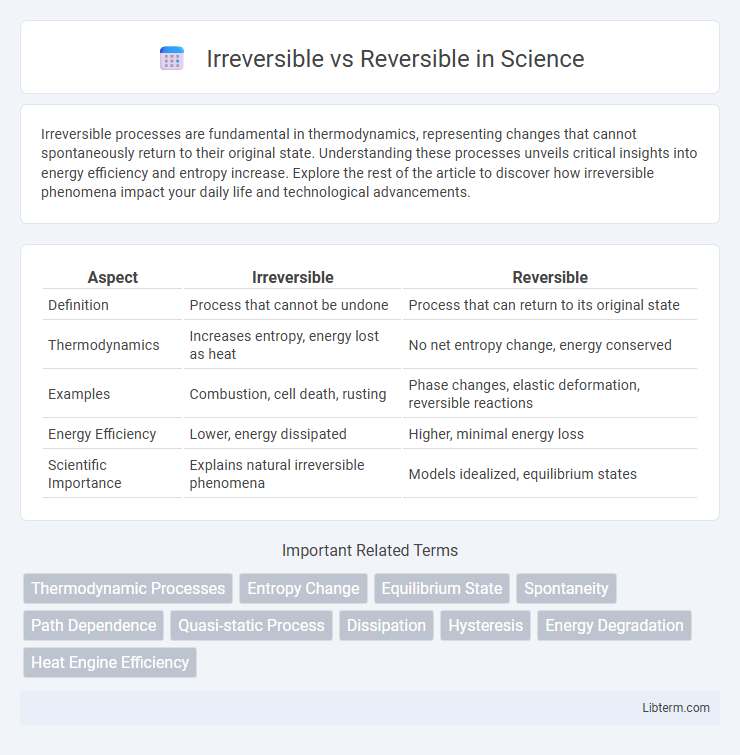
Table of Comparison
| Aspect | Irreversible | Reversible |
|---|---|---|
| Definition | Process that cannot be undone | Process that can return to its original state |
| Thermodynamics | Increases entropy, energy lost as heat | No net entropy change, energy conserved |
| Examples | Combustion, cell death, rusting | Phase changes, elastic deformation, reversible reactions |
| Energy Efficiency | Lower, energy dissipated | Higher, minimal energy loss |
| Scientific Importance | Explains natural irreversible phenomena | Models idealized, equilibrium states |
Understanding Irreversible and Reversible Processes
Irreversible processes involve changes that cannot return a system to its original state without external intervention, often accompanied by energy dissipation or entropy increase, such as friction or spontaneous heat transfer. Reversible processes occur in idealized systems where changes happen quasi-statically, allowing the system to be restored to its initial condition without entropy production or loss of energy. Understanding these concepts is crucial in thermodynamics for analyzing system efficiency, energy conservation, and predicting natural process behavior.
Key Differences Between Irreversible and Reversible
Irreversible processes result in permanent changes where the system cannot return to its original state, while reversible processes allow the system to restore its initial conditions without energy loss or entropy increase. Reversible changes occur under idealized conditions with infinitesimally slow transformations, preserving equilibrium throughout the process. Key differences include the presence of entropy production in irreversible processes and the exact retracing of states in reversible processes.
Examples of Irreversible in Daily Life
Burning paper and cooking an egg are common examples of irreversible processes, where the original substances cannot be restored to their initial state. Rusting of iron and souring of milk demonstrate chemical changes that produce new substances, making reversal impossible. These irreversible changes contrast with reversible processes like melting ice, highlighting permanent transformations in daily life.
Examples of Reversible in Daily Life
Reversible processes in daily life include melting ice, boiling water, and freezing liquids, where the initial state can be restored without significant changes to the system. Dissolving sugar in water is another example, as the sugar can be recovered by evaporating the water. These reversible changes contrast with irreversible ones, which involve permanent transformations, such as burning paper or cooking an egg.
Thermodynamics: Reversible vs Irreversible Processes
Reversible processes in thermodynamics occur infinitely slowly, maintaining equilibrium at every stage, resulting in no entropy generation and maximum efficiency. Irreversible processes involve finite rates, friction, or unbalanced forces, causing entropy to increase and energy to dissipate as waste heat. The distinction between reversible and irreversible processes is fundamental in determining the efficiency limits of engines and refrigerators in thermodynamic cycles.
Chemical Reactions: Reversible vs Irreversible
Irreversible chemical reactions proceed to completion, forming products that do not readily convert back to reactants, typically characterized by a large negative Gibbs free energy change. Reversible chemical reactions establish an equilibrium state where forward and reverse reaction rates are equal, allowing continuous interconversion between reactants and products. Equilibrium constants (K_eq) and reaction conditions such as temperature and pressure significantly influence the extent to which reversible reactions favor reactants or products.
Impact on Energy Efficiency
Irreversible processes result in energy dissipation, reducing overall efficiency due to entropy generation and loss of usable work. Reversible processes ideally maintain total energy, enabling maximum efficiency by minimizing entropy changes and energy waste. Industrial systems designed for reversibility achieve superior energy conservation and cost savings by limiting thermodynamic irreversibilities.
Factors Influencing Irreversibility
Irreversibility is primarily influenced by factors such as entropy production, energy dissipation, and the system's deviation from thermodynamic equilibrium. Processes involving high friction, rapid changes, or chemical reactions with substantial activation energy barriers tend to increase irreversibility. Understanding these factors is crucial in thermodynamics, engineering, and physical chemistry for optimizing efficiency and predicting system behavior.
Practical Applications and Implications
Irreversible processes, common in engines and chemical reactions, lead to energy loss as heat, impacting efficiency in practical applications such as power plants and manufacturing. Reversible processes, idealized in thermodynamics, offer maximum work output with minimal entropy change, guiding the design of efficient engines, refrigerators, and heat pumps. Understanding the distinction helps optimize energy systems, reduce waste, and improve sustainability in industrial and environmental contexts.
Summary: Choosing Between Irreversible and Reversible
Choosing between irreversible and reversible processes depends on factors like energy efficiency, system sustainability, and control over outcomes. Irreversible processes often result in energy loss and less precise control, ideal for single-use scenarios. Reversible processes maximize energy conservation and allow for system recovery, making them preferred in sustainable and repeatable applications.
Irreversible Infographic
 libterm.com
libterm.com