Reductive approaches simplify complex ideas by breaking them down into basic components, often overlooking essential nuances that contribute to full understanding. This method can streamline problem-solving but may lead to incomplete conclusions if critical details are ignored. Explore the rest of the article to discover how reductive thinking impacts various fields and when to apply it effectively.
Table of Comparison
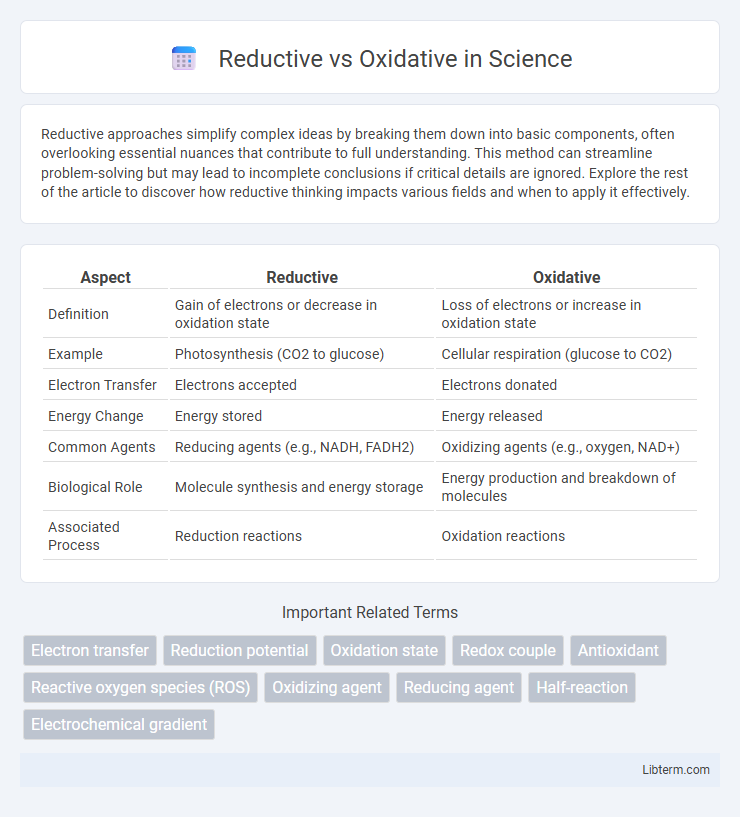
| Aspect | Reductive | Oxidative |
|---|---|---|
| Definition | Gain of electrons or decrease in oxidation state | Loss of electrons or increase in oxidation state |
| Example | Photosynthesis (CO2 to glucose) | Cellular respiration (glucose to CO2) |
| Electron Transfer | Electrons accepted | Electrons donated |
| Energy Change | Energy stored | Energy released |
| Common Agents | Reducing agents (e.g., NADH, FADH2) | Oxidizing agents (e.g., oxygen, NAD+) |
| Biological Role | Molecule synthesis and energy storage | Energy production and breakdown of molecules |
| Associated Process | Reduction reactions | Oxidation reactions |
Introduction to Reductive and Oxidative Processes
Reductive and oxidative processes are fundamental biochemical reactions involving electron transfer, where reductive processes gain electrons and oxidative processes lose electrons. Redox reactions play a critical role in cellular metabolism, energy production, and biochemical synthesis, with molecules like NADH and FADH2 acting as key electron carriers. Understanding these processes is essential for studying cellular respiration, photosynthesis, and metabolic regulation.
Defining Reductive Reactions
Reductive reactions involve the gain of electrons or a decrease in oxidation state by a molecule, atom, or ion during a chemical process. These reactions are critical in cellular metabolism, particularly in pathways like the Calvin cycle where carbon dioxide is reduced to glucose. Reductive processes often pair with oxidative reactions, forming redox reactions essential for energy transfer in biological systems.
Understanding Oxidative Reactions
Oxidative reactions involve the loss of electrons from a molecule, atom, or ion, often resulting in the increase of oxidation state. These reactions are crucial in cellular respiration where glucose is oxidized to produce energy in the form of ATP. Key players in oxidative processes include oxygen as the ultimate electron acceptor and enzymes like oxidases and dehydrogenases that facilitate electron transfer.
Key Differences Between Reduction and Oxidation
Reduction involves the gain of electrons, resulting in a decrease in oxidation state, while oxidation entails the loss of electrons and an increase in oxidation state. In biochemical processes, reductive reactions typically add hydrogen or remove oxygen, whereas oxidative reactions add oxygen or remove hydrogen. Redox reactions are essential in cellular respiration and photosynthesis, driving energy transfer through electron exchange.
Role of Electrons in Reductive vs Oxidative Reactions
Reductive reactions involve the gain of electrons by a molecule, atom, or ion, resulting in a decrease in oxidation state. Oxidative reactions entail the loss of electrons, causing an increase in oxidation state and often producing energy in cellular processes like respiration. The transfer of electrons in these reactions is fundamental to energy conversion and metabolic pathways in biochemistry.
Biological Examples: Reductive and Oxidative Pathways
Reductive pathways in biology often involve the conversion of molecules to more reduced forms, exemplified by the Calvin cycle where carbon dioxide is fixed into glucose. Oxidative pathways, such as cellular respiration, break down glucose into carbon dioxide and water while releasing energy stored in ATP. Both pathways are essential for maintaining cellular energy balance and biosynthesis.
Industrial Applications of Reductive and Oxidative Chemistry
Reductive and oxidative chemistries are fundamental to industrial applications such as pharmaceuticals, petrochemicals, and metallurgy, where reductive processes enable the synthesis of amines and alcohols through hydrogenation, while oxidative reactions facilitate the production of acids, aldehydes, and epoxides via controlled oxidation. Industries rely on reductive chemistry for catalytic hydrogenation in refining crude oil and manufacturing fertilizers, whereas oxidative chemistry is crucial in the production of intermediates like terephthalic acid for polymers and the detoxification of pollutants through advanced oxidation processes. The choice between reductive and oxidative methods depends on desired chemical transformations, efficiency, and environmental impact, making both essential for sustainable industrial development.
Redox Reactions: The Interplay of Reduction and Oxidation
Redox reactions involve the simultaneous process of reduction, where a substance gains electrons, and oxidation, where another loses electrons, facilitating electron transfer and energy exchange in chemical systems. Reduction is characterized by the decrease in oxidation state, often involving the addition of hydrogen or removal of oxygen, while oxidation entails an increase in oxidation state through the loss of electrons or addition of oxygen. This dynamic interplay underpins essential biological processes like cellular respiration and photosynthesis, as well as industrial applications such as corrosion prevention and energy production.
Measuring Reductive and Oxidative Strength
Measuring reductive and oxidative strength involves evaluating the electron-donating and electron-accepting abilities of substances using redox potential, expressed in volts (V) against a standard hydrogen electrode. Techniques like cyclic voltammetry provide detailed insights into oxidation-reduction behavior by recording current responses to applied voltage sweeps, enabling accurate assessment of a compound's redox capacity. Quantitative analysis of redox potential supports the prediction of reaction feasibility and the design of materials for energy storage, catalysis, and biochemical applications.
Summary: Choosing Between Reductive and Oxidative Approaches
Choosing between reductive and oxidative approaches depends on the desired chemical transformation and energy efficiency. Reductive methods often involve the gain of electrons, useful for processes such as hydrogenation and reduction of metal oxides, while oxidative methods focus on electron loss, critical for reactions like combustion and oxidation of organic compounds. Selecting the appropriate approach requires evaluating reaction conditions, catalyst availability, and target product requirements to maximize yield and sustainability.
Reductive Infographic
 libterm.com
libterm.com