Inverse functions reverse the effect of the original function, mapping outputs back to their corresponding inputs. Understanding the properties and applications of inverse functions is crucial in fields like algebra, calculus, and computer science. Explore the rest of the article to deepen your grasp of how inverse functions operate and their practical uses.
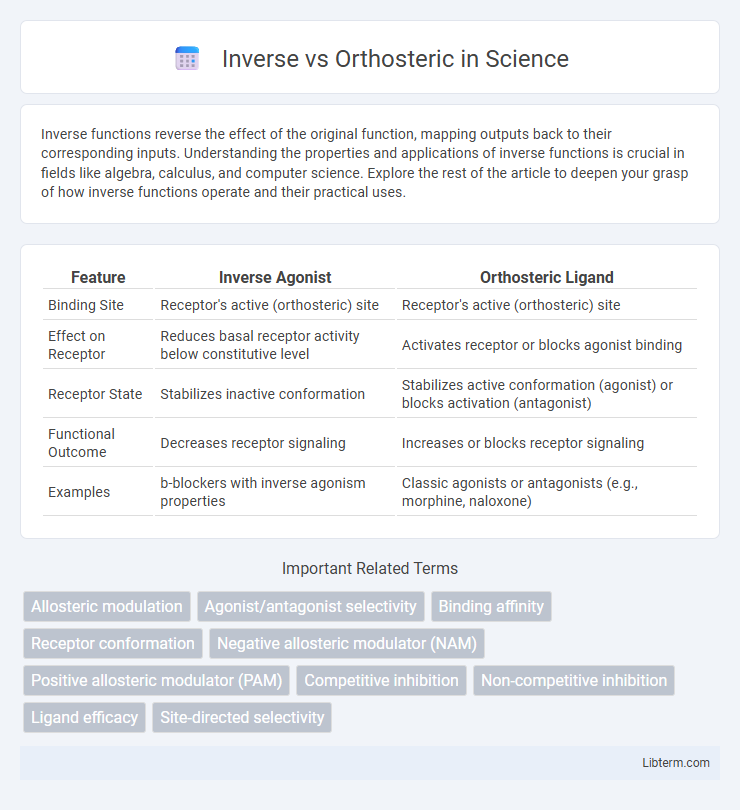
Table of Comparison
| Feature | Inverse Agonist | Orthosteric Ligand |
|---|---|---|
| Binding Site | Receptor's active (orthosteric) site | Receptor's active (orthosteric) site |
| Effect on Receptor | Reduces basal receptor activity below constitutive level | Activates receptor or blocks agonist binding |
| Receptor State | Stabilizes inactive conformation | Stabilizes active conformation (agonist) or blocks activation (antagonist) |
| Functional Outcome | Decreases receptor signaling | Increases or blocks receptor signaling |
| Examples | b-blockers with inverse agonism properties | Classic agonists or antagonists (e.g., morphine, naloxone) |
Introduction to Ligand Binding: Inverse vs Orthosteric
Orthosteric ligands bind directly to the primary active site of a receptor, modulating its natural response by either activating or blocking the receptor. Inverse agonists also bind at the orthosteric site but reduce the basal activity of the receptor, producing effects opposite to agonists. Understanding these ligand-receptor interactions is critical for drug design targeting receptor-mediated signaling pathways.
Defining Orthosteric Binding Sites
Orthosteric binding sites are the primary active regions on receptors where endogenous ligands such as neurotransmitters or hormones bind, triggering a biological response. These sites are highly specific and are the targets for many conventional agonists and antagonists, differentiating them from allosteric or inverse binding sites. Understanding the structural conformation of orthosteric sites is crucial for drug design, as it informs the development of molecules that can either activate or inhibit receptor function effectively.
Understanding Inverse Ligands and Their Mechanisms
Inverse ligands bind to the same orthosteric site as agonists but induce the opposite effect by stabilizing the receptor's inactive conformation. Unlike orthosteric ligands that activate receptors, inverse ligands reduce basal activity and decrease downstream signaling, playing a critical role in modulating receptor function. Exploring inverse ligand mechanisms provides insights into receptor pharmacology and the development of drugs targeting constitutively active receptors.
Structural Differences in Binding: Orthosteric vs Inverse
Orthosteric binding involves ligands interacting directly with the primary active site of a receptor, typically stabilizing the receptor in an active conformation. Inverse agonists bind to the same orthosteric site but induce a distinct conformational change that favors the receptor's inactive state, reducing basal activity. Structural studies reveal that inverse agonists often form unique hydrogen bonds and steric interactions that shift receptor domains differently compared to orthosteric agonists, highlighting the nuanced differences in receptor modulation at the molecular level.
Pharmacological Effects: Contrasts Between Inverse and Orthosteric Ligands
Inverse agonists bind to the same receptor site as orthosteric ligands but elicit pharmacological effects by stabilizing the receptor in its inactive conformation, leading to decreased basal activity, while orthosteric agonists activate the receptor to produce a biological response. Orthosteric antagonists competitively inhibit receptor activation by blocking the binding site without intrinsic activity, contrasting with inverse agonists that actively reduce receptor constitutive signaling. These differences profoundly impact therapeutic applications, affecting receptor signaling pathways, drug efficacy, and side effect profiles.
Therapeutic Implications of Orthosteric Modulators
Orthosteric modulators bind directly to the active site of receptors, offering targeted modulation of physiological responses with established specificity, which can enhance therapeutic efficacy and reduce off-target effects. Unlike inverse agonists that reduce constitutive receptor activity, orthosteric agonists or antagonists provide predictable dose-dependent outcomes crucial for treating conditions like hypertension, asthma, and neurodegenerative diseases. The clinical application of orthosteric modulators enables precise receptor activity control, facilitating drug design focused on maximizing efficacy and minimizing adverse reactions.
Clinical Relevance of Inverse Ligands
Inverse ligands bind to the same orthosteric site as agonists but induce opposite receptor conformations, leading to decreased receptor activity, which is crucial in modulating excessive signaling pathways in diseases. Clinically, inverse agonists are prominent in treating conditions like anxiety, schizophrenia, and certain cancers by stabilizing receptors in inactive states, thereby reducing pathological signaling. Unlike orthosteric antagonists that block receptor activation without altering constitutive activity, inverse ligands offer enhanced therapeutic specificity by suppressing basal receptor activity, improving efficacy and minimizing side effects.
Advantages and Challenges in Drug Development
Inverse agonists offer the advantage of reducing constitutive receptor activity, providing therapeutic benefits in conditions where receptor overactivation is implicated, whereas orthosteric ligands directly compete with endogenous agonists, often leading to more predictable pharmacological profiles. Challenges in inverse agonist development include achieving selectivity and avoiding off-target effects due to receptor isoform variability, while orthosteric drug development faces difficulties linked to competition with high concentrations of natural ligands and potential desensitization. Exploiting allosteric sites or designing subtype-selective inverse agonists can overcome limitations associated with orthosteric binding, but require extensive screening and structural understanding to optimize efficacy and safety.
Recent Advances in Ligand Design and Targeting
Recent advances in ligand design have enabled precise differentiation between inverse and orthosteric ligands, enhancing selective targeting of G protein-coupled receptors (GPCRs). Structural insights from high-resolution crystallography reveal that inverse agonists stabilize inactive receptor conformations, while orthosteric ligands compete directly at endogenous binding sites. Emerging allosteric modulators and biased ligands offer innovative strategies to achieve receptor subtype specificity and improved therapeutic efficacy.
Future Directions in Orthosteric and Inverse Ligand Research
Future directions in orthosteric and inverse ligand research emphasize the development of highly selective compounds targeting G protein-coupled receptors (GPCRs) to improve therapeutic efficacy and reduce side effects. Advances in structural biology and computational modeling enable precise modulation of receptor conformations, enhancing the design of inverse agonists that stabilize inactive receptor states versus orthosteric ligands that compete at primary binding sites. Integration of artificial intelligence and high-throughput screening accelerates the identification of novel ligands, fostering personalized medicine approaches and expanding treatment options for complex diseases.
Inverse Infographic
 libterm.com
libterm.com