Filtration is a critical process used to remove impurities and particles from liquids or gases, improving quality and safety. Efficient filtration techniques enhance the performance of industrial systems, protect equipment, and ensure clean water and air. Explore the rest of the article to discover various filtration methods and how they can benefit your applications.
Table of Comparison
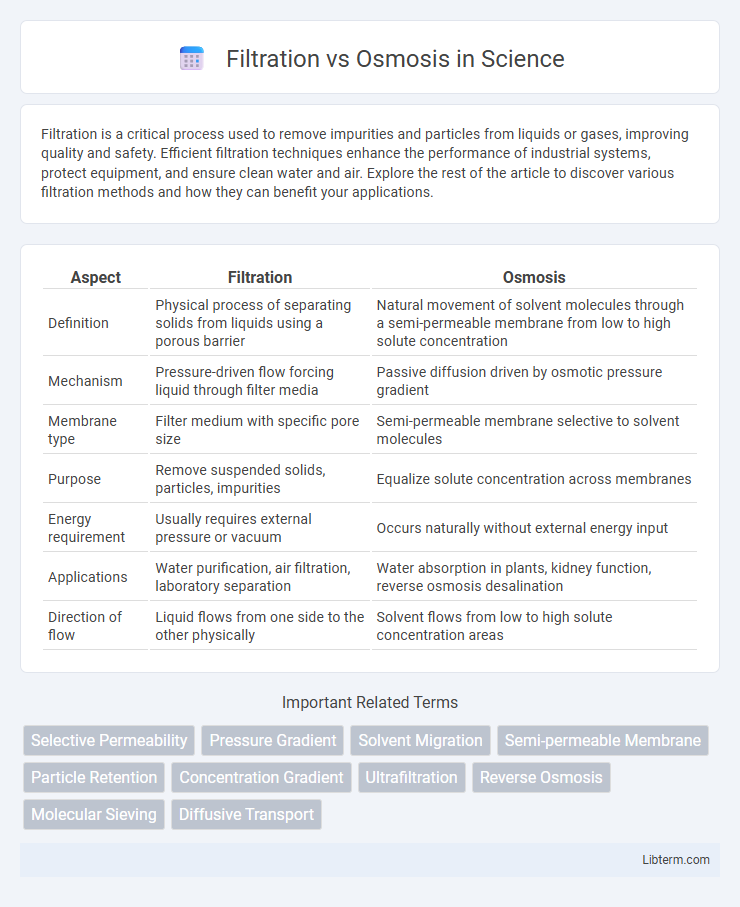
| Aspect | Filtration | Osmosis |
|---|---|---|
| Definition | Physical process of separating solids from liquids using a porous barrier | Natural movement of solvent molecules through a semi-permeable membrane from low to high solute concentration |
| Mechanism | Pressure-driven flow forcing liquid through filter media | Passive diffusion driven by osmotic pressure gradient |
| Membrane type | Filter medium with specific pore size | Semi-permeable membrane selective to solvent molecules |
| Purpose | Remove suspended solids, particles, impurities | Equalize solute concentration across membranes |
| Energy requirement | Usually requires external pressure or vacuum | Occurs naturally without external energy input |
| Applications | Water purification, air filtration, laboratory separation | Water absorption in plants, kidney function, reverse osmosis desalination |
| Direction of flow | Liquid flows from one side to the other physically | Solvent flows from low to high solute concentration areas |
Introduction to Filtration and Osmosis
Filtration is a physical process that separates particles from a fluid by passing it through a porous material, effectively removing impurities based on particle size. Osmosis is a natural diffusion process where solvent molecules move across a semi-permeable membrane from a region of lower solute concentration to higher solute concentration, aiming to equalize concentrations. Both techniques are fundamental in water purification, with filtration primarily eliminating suspended solids and osmosis targeting dissolved solutes.
Defining Filtration: Processes and Principles
Filtration involves the mechanical or physical process of separating solid particles from liquids or gases by passing the mixture through a porous medium or filter, relying on particle size and physical barriers to achieve separation. Key principles include the use of filter media with specific pore sizes, pressure differences to drive fluid flow, and retention mechanisms such as sieving, diffusion, or adsorption. This process is widely used in water treatment, air purification, and chemical processing to remove impurities without altering the chemical composition of the filtered substance.
Understanding Osmosis: Mechanisms and Key Concepts
Osmosis is the passive movement of water molecules across a semipermeable membrane from a region of low solute concentration to high solute concentration, driven by osmotic pressure. This natural process balances solute concentrations on both sides of the membrane without requiring energy input. Understanding key concepts such as water potential, membrane permeability, and osmotic gradients is essential for distinguishing osmosis from filtration, which involves pressure-driven solute separation.
Types of Filtration Techniques
Filtration techniques vary widely and include microfiltration, ultrafiltration, nanofiltration, and reverse osmosis, each designed for specific particle size removal and applications. Microfiltration removes particles ranging from 0.1 to 10 microns, commonly used in water purification and food processing. Ultrafiltration and nanofiltration further refine contaminants at molecular levels, while reverse osmosis employs a semi-permeable membrane to eliminate dissolved solutes, making it distinct from typical filtration methods.
Types of Osmosis: Forward and Reverse Osmosis
Forward osmosis utilizes a natural osmotic pressure gradient to pass water through a semi-permeable membrane, separating contaminants without requiring external pressure, making it energy-efficient for hydration and wastewater treatment. Reverse osmosis employs applied pressure to force water through a membrane, effectively removing dissolved salts, bacteria, and other impurities, commonly used in desalination and water purification systems. Both types leverage osmotic principles but differ in energy usage and application scope.
Applications of Filtration in Everyday Life
Filtration is widely applied in everyday life for water purification, air filtration in HVAC systems, and coffee brewing, where it removes impurities, particulates, and contaminants to improve quality and safety. Household water filters utilize materials like activated carbon and ceramic to eliminate bacteria, chlorine, and sediments, enhancing taste and health. Air filtration systems capture dust, pollen, and allergens, promoting cleaner indoor air and reducing respiratory issues for occupants.
Real-World Uses of Osmosis
Osmosis plays a critical role in water purification systems, especially in reverse osmosis filters that remove contaminants and provide clean drinking water. Agricultural irrigation benefits from osmosis by enabling efficient water absorption in plant roots, promoting healthier crop growth. Medical treatments such as dialysis employ osmosis principles to filter waste products from the blood when kidney function is impaired.
Key Differences Between Filtration and Osmosis
Filtration separates particles from a fluid using a physical barrier or filter based on particle size, while osmosis involves the movement of solvent molecules through a semi-permeable membrane from low to high solute concentration. Filtration relies on pressure differences to push fluid through the filter, whereas osmosis depends on osmotic pressure driven by solute concentration gradients. The primary function of filtration is mechanical separation of solids from liquids, in contrast to osmosis, which regulates solvent balance in biological and chemical processes.
Advantages and Limitations of Each Method
Filtration effectively removes suspended solids and larger particles, offering quick processing and low energy consumption but struggles to eliminate dissolved contaminants and microorganisms. Osmosis, particularly reverse osmosis, excels at removing dissolved salts, heavy metals, and pathogens, providing high purity water but requires significant pressure, high energy input, and frequent membrane maintenance. Filtration suits pre-treatment and turbidity removal, while osmosis is preferred for desalination and removal of microscopic contaminants, balancing efficiency with cost and operational complexity.
Choosing the Right Process: Filtration or Osmosis
Choosing between filtration and osmosis depends on the specific application and desired purity level. Filtration effectively removes larger particles and contaminants using physical barriers, ideal for water pre-treatment and industrial processes. Osmosis, particularly reverse osmosis, provides superior removal of dissolved salts and microscopic impurities, making it essential for producing ultrapure water in medical and laboratory settings.
Filtration Infographic
 libterm.com
libterm.com