Electrolytes are essential minerals that carry an electric charge, crucial for maintaining fluid balance, nerve function, and muscle contractions in your body. Common electrolytes include sodium, potassium, calcium, and magnesium, each playing a specific role in hydration and overall health. Discover how optimizing your electrolyte levels can enhance performance and well-being by reading the rest of the article.
Table of Comparison
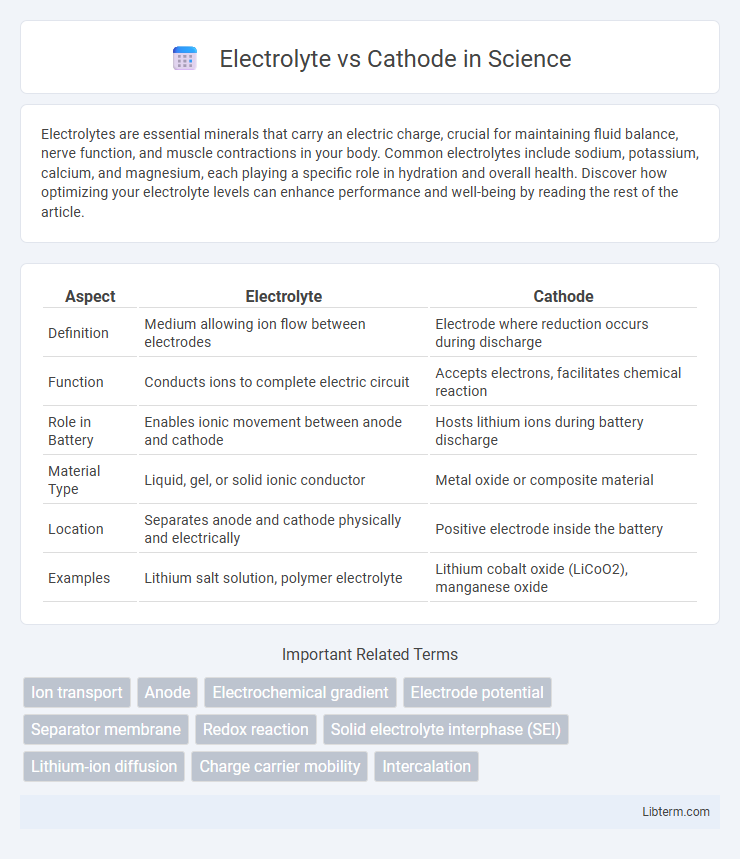
| Aspect | Electrolyte | Cathode |
|---|---|---|
| Definition | Medium allowing ion flow between electrodes | Electrode where reduction occurs during discharge |
| Function | Conducts ions to complete electric circuit | Accepts electrons, facilitates chemical reaction |
| Role in Battery | Enables ionic movement between anode and cathode | Hosts lithium ions during battery discharge |
| Material Type | Liquid, gel, or solid ionic conductor | Metal oxide or composite material |
| Location | Separates anode and cathode physically and electrically | Positive electrode inside the battery |
| Examples | Lithium salt solution, polymer electrolyte | Lithium cobalt oxide (LiCoO2), manganese oxide |
Introduction: Defining Electrolyte and Cathode
An electrolyte is a medium containing ions that facilitates the flow of electric charge between the cathode and anode in a battery or electrochemical cell. The cathode serves as the positive electrode where reduction reactions occur during the discharge cycle, attracting electrons from the external circuit. Understanding the distinct roles of electrolytes and cathodes is essential for optimizing battery performance and efficiency.
Key Roles in Electrochemical Cells
Electrolytes serve as ion conductors in electrochemical cells, enabling ion transport between the cathode and anode to maintain charge balance during redox reactions. Cathodes act as the site for reduction reactions, accepting electrons and facilitating the storage or release of energy depending on the cell's operation mode. The synergy between electrolytes and cathodes governs the efficiency, capacity, and durability of batteries and fuel cells.
Composition and Material Differences
Electrolytes are typically composed of ionic compounds dissolved in solvents, such as lithium salts in organic solvents for lithium-ion batteries, enabling ionic conductivity between electrodes. Cathodes consist of complex metal oxides like lithium cobalt oxide (LiCoO2) or lithium iron phosphate (LiFePO4) that serve as the source of lithium ions during discharge. While electrolytes facilitate ion transport through liquid or solid mediums, cathodes provide the structural framework and electrochemical activity for lithium-ion intercalation and electron transfer.
Functionality in Battery Architectures
Electrolytes facilitate ion transport between the cathode and anode, ensuring efficient charge transfer and overall battery performance. The cathode serves as the positive electrode where reduction reactions occur, storing and releasing lithium ions during discharge and charge cycles. Effective battery architectures rely on the interplay between electrolyte conductivity and cathode material stability to optimize energy density and cycle life.
Electrolyte: Types and Characteristics
Electrolytes in batteries serve as the medium for ion transport between the cathode and anode, crucial for energy storage and release. Common types of electrolytes include liquid electrolytes, solid-state electrolytes, and gel polymer electrolytes, each with distinct characteristics such as ionic conductivity, thermal stability, and electrochemical window. Liquid electrolytes typically offer high ionic conductivity but face leakage and volatility issues, whereas solid-state electrolytes provide enhanced safety and mechanical stability, with ongoing research aimed at improving their conductivity for advanced battery applications.
Cathode: Types and Materials
Cathodes in batteries are crucial for determining energy density and lifespan, with common types including layered oxide, spinel, and polyanion cathodes. Layered oxide cathodes, such as lithium cobalt oxide (LiCoO2) and lithium nickel manganese cobalt oxide (NMC), offer high energy density and are widely used in lithium-ion batteries. Spinel cathodes like lithium manganese oxide (LiMn2O4) provide structural stability and high power capability, whereas polyanion cathodes such as lithium iron phosphate (LiFePO4) are renowned for thermal stability and long cycle life.
Importance to Battery Performance
Electrolytes play a crucial role in battery performance by enabling efficient ion transport between the cathode and anode, directly affecting charge and discharge rates. The cathode's material composition determines energy density and voltage output, making its optimization vital for high-capacity batteries. Balanced interaction between the electrolyte and cathode ensures battery stability, longevity, and overall efficiency in energy storage applications.
Electrolyte vs Cathode: Safety Considerations
Electrolytes and cathodes differ significantly in safety considerations, with electrolytes often posing risks of leakage, flammability, and chemical instability in lithium-ion batteries. Solid-state electrolytes enhance safety by reducing the risk of dendrite formation and thermal runaway compared to liquid electrolytes. Cathode materials such as lithium cobalt oxide (LCO) or lithium nickel manganese cobalt oxide (NMC) influence battery thermal stability and toxicity, impacting overall device safety during operation and failure scenarios.
Impact on Energy Density and Lifespan
The electrolyte's conductivity and stability directly affect ion transport efficiency and battery lifespan, while the cathode's material composition determines the maximum energy density and voltage output. High-performance cathodes like lithium nickel manganese cobalt oxide (NMC) can increase energy density significantly, but require compatible electrolytes to maintain cycle stability and prevent degradation. Optimizing the interaction between electrolyte and cathode materials enhances overall battery capacity and extends operational lifespan by reducing side reactions and capacity fade.
Future Trends in Electrolyte and Cathode Development
Future trends in electrolyte development emphasize solid-state electrolytes offering higher ionic conductivity and enhanced safety for lithium-ion and beyond-lithium batteries. Cathode research is advancing toward high-capacity materials such as high-nickel layered oxides and sulfur-based compounds to increase energy density and cycle life. Integration of electrolyte and cathode innovations aims to optimize interfacial stability and overall battery performance for next-generation energy storage systems.
Electrolyte Infographic
 libterm.com
libterm.com