Transesterification is a chemical process that involves exchanging the organic group R'' of an ester with the organic group R' of an alcohol. This reaction is essential in biodiesel production, converting fats and oils into usable fuel by reacting them with alcohol. Explore the rest of the article to understand how transesterification can optimize your renewable energy projects.
Table of Comparison
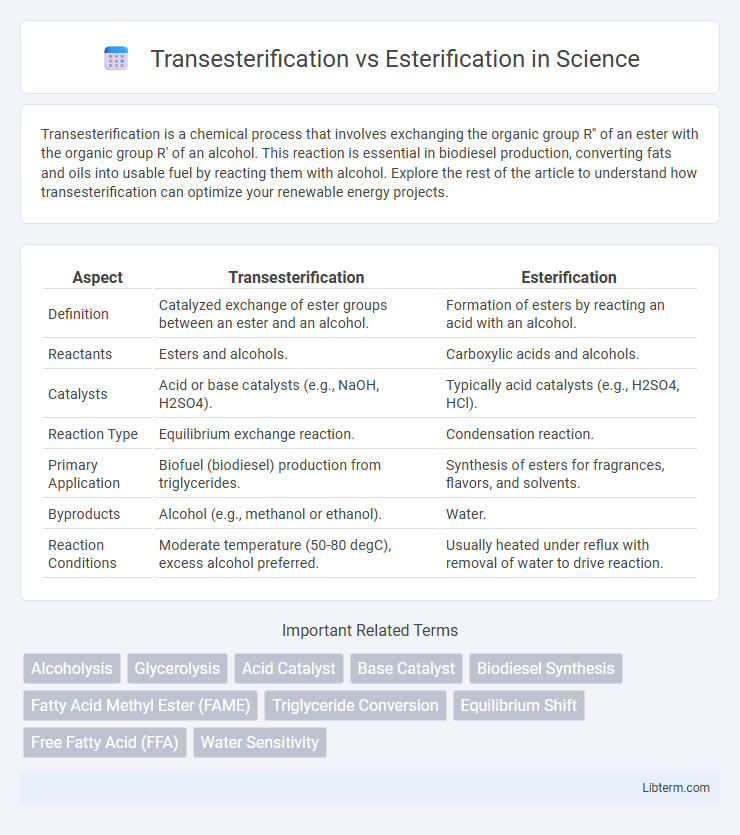
| Aspect | Transesterification | Esterification |
|---|---|---|
| Definition | Catalyzed exchange of ester groups between an ester and an alcohol. | Formation of esters by reacting an acid with an alcohol. |
| Reactants | Esters and alcohols. | Carboxylic acids and alcohols. |
| Catalysts | Acid or base catalysts (e.g., NaOH, H2SO4). | Typically acid catalysts (e.g., H2SO4, HCl). |
| Reaction Type | Equilibrium exchange reaction. | Condensation reaction. |
| Primary Application | Biofuel (biodiesel) production from triglycerides. | Synthesis of esters for fragrances, flavors, and solvents. |
| Byproducts | Alcohol (e.g., methanol or ethanol). | Water. |
| Reaction Conditions | Moderate temperature (50-80 degC), excess alcohol preferred. | Usually heated under reflux with removal of water to drive reaction. |
Introduction to Esterification and Transesterification
Esterification is a chemical reaction between an acid, typically a carboxylic acid, and an alcohol, producing an ester and water, often catalyzed by acids. Transesterification involves exchanging the organic group of an ester with the organic group of an alcohol, commonly used in biodiesel production by converting triglycerides and methanol into methyl esters and glycerol. Both processes are crucial in organic synthesis, impacting industries such as pharmaceuticals, plastics, and biofuels, with esterification focusing on ester formation from acids and alcohols, while transesterification modifies existing esters.
Chemical Principles of Esterification
Esterification is a chemical reaction between an acid, typically a carboxylic acid, and an alcohol, resulting in the formation of an ester and water through a condensation process catalyzed by acid. Transesterification involves the exchange of the alkoxy group of an ester with another alcohol, producing a different ester and alcohol, commonly used in biodiesel production. The fundamental chemical principle of esterification lies in the protonation of the carbonyl oxygen, activation of the carbonyl carbon, nucleophilic attack by the alcohol, and subsequent removal of water to form the ester bond.
Mechanism of Transesterification Reactions
Transesterification involves the exchange of alkoxy groups between an ester and an alcohol, facilitated by acid or base catalysts, resulting in the formation of a new ester and alcohol. The mechanism begins with nucleophilic attack by the alcohol on the ester carbonyl carbon, forming a tetrahedral intermediate that collapses to release the original alcohol and generate the transesterified product. This reaction contrasts with esterification, where a carboxylic acid reacts with an alcohol to produce an ester and water through protonation and dehydration steps.
Key Differences Between Esterification and Transesterification
Esterification is a chemical reaction between an acid and an alcohol producing an ester and water, commonly used in synthesizing esters from carboxylic acids and alcohols. Transesterification involves exchanging the organic group R'' of an ester with the organic group R' of an alcohol, primarily used to modify esters or produce biodiesel from triglycerides and methanol. Key differences include reactants--esterification uses an acid and alcohol, transesterification uses an ester and alcohol--and byproducts, as esterification releases water while transesterification produces a different ester and alcohol.
Catalysts Used in Both Processes
Transesterification primarily utilizes alkali catalysts such as sodium hydroxide (NaOH) or potassium hydroxide (KOH) due to their high efficiency in converting triglycerides into biodiesel and glycerol. Esterification typically employs acid catalysts like sulfuric acid (H2SO4) to effectively transform free fatty acids into esters, especially in feedstocks with high acid content. Enzymatic catalysts, including lipases, are increasingly explored in both processes for their specificity and environmentally friendly profiles, although they present higher costs and slower reaction rates.
Industrial Applications and Relevance
Transesterification is a critical industrial process primarily used in biodiesel production, where triglycerides react with alcohols to form fatty acid alkyl esters and glycerol, optimizing renewable energy generation. Esterification, involving the direct acid-catalyzed reaction between carboxylic acids and alcohols to produce esters and water, is essential in manufacturing pharmaceuticals, fragrances, and plasticizers. The choice between transesterification and esterification depends on feedstock availability, desired product properties, and industrial scalability, impacting sectors like biofuels, cosmetics, and polymer production.
Advantages and Limitations of Each Method
Transesterification offers a faster reaction rate and higher yield of biodiesel compared to esterification, making it ideal for converting triglycerides in vegetable oils into fatty acid methyl esters. However, transesterification requires alkaline catalysts and is sensitive to free fatty acid content, which can lead to soap formation and complicate product separation. Esterification, on the other hand, effectively reduces high free fatty acid levels in feedstocks using acid catalysts, but it is slower and less efficient for producing biodiesel directly from triglycerides.
Factors Affecting Reaction Efficiency
Transesterification reaction efficiency depends heavily on catalyst concentration, temperature, and alcohol-to-oil molar ratio, with alkaline catalysts like NaOH or KOH commonly accelerating the process. Esterification efficiency is influenced by the acid catalyst concentration, reaction temperature, and molar ratio of alcohol to free fatty acids, where sulfuric acid often serves as a catalyst to convert free fatty acids into esters. Both reactions require precise control of reaction time and water content to minimize hydrolysis and maximize product yield in biodiesel production.
Environmental Impact and Sustainability
Transesterification generally produces biodiesel from renewable feedstocks like vegetable oils and animal fats, offering a more sustainable alternative to fossil fuels by reducing greenhouse gas emissions. Esterification typically involves the conversion of free fatty acids into esters but can generate more waste and requires acidic catalysts, making it less environmentally friendly. The widespread adoption of transesterification for biodiesel aligns with circular economy principles, promoting resource efficiency and reduced carbon footprint compared to traditional esterification processes.
Conclusion: Choosing the Right Process
Selecting between transesterification and esterification depends largely on the desired product and feedstock characteristics; transesterification suits biodiesel production from triglycerides, while esterification is ideal for synthesizing esters from free fatty acids or small alcohols. Efficiency factors include reaction conditions, catalyst type, and feedstock purity, with acid catalysts favored in esterification for free fatty acid conversion and base catalysts common in transesterification for triglyceride conversion. Understanding the chemical feedstock composition and target ester properties ensures the optimal choice of process for industrial applications.
Transesterification Infographic
 libterm.com
libterm.com