Additivity refers to the principle where the combined effect of multiple factors equals the sum of their individual effects, commonly used in fields like mathematics, chemistry, and statistics. Understanding this concept helps improve your ability to analyze complex systems by breaking them down into simpler, additive components. Discover how additivity impacts various applications by exploring the rest of the article.
Table of Comparison
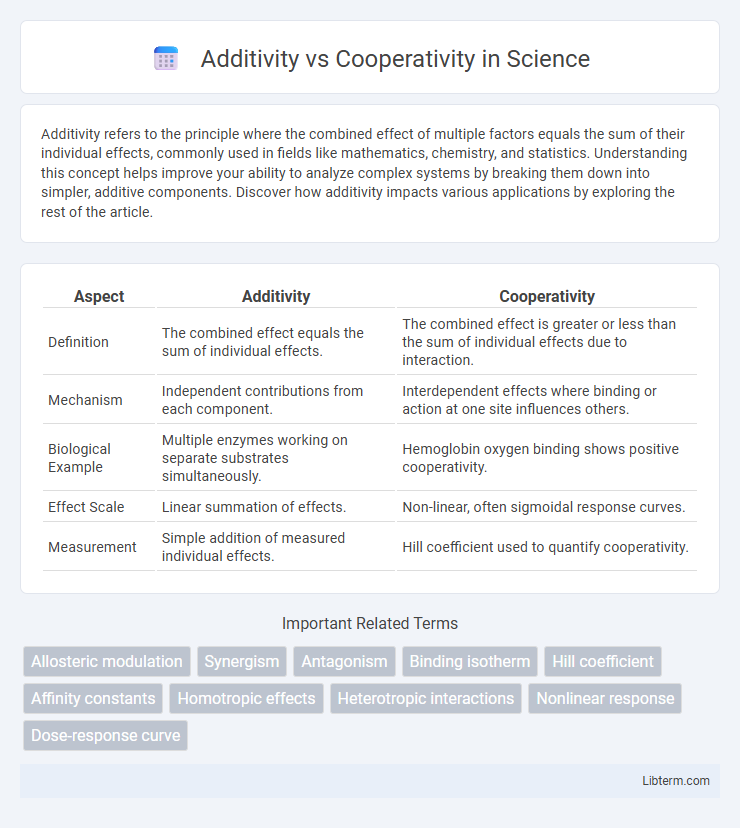
| Aspect | Additivity | Cooperativity |
|---|---|---|
| Definition | The combined effect equals the sum of individual effects. | The combined effect is greater or less than the sum of individual effects due to interaction. |
| Mechanism | Independent contributions from each component. | Interdependent effects where binding or action at one site influences others. |
| Biological Example | Multiple enzymes working on separate substrates simultaneously. | Hemoglobin oxygen binding shows positive cooperativity. |
| Effect Scale | Linear summation of effects. | Non-linear, often sigmoidal response curves. |
| Measurement | Simple addition of measured individual effects. | Hill coefficient used to quantify cooperativity. |
Understanding Additivity and Cooperativity
Additivity refers to the independent effects of individual components summing to produce a total response without influencing each other, commonly seen in enzyme kinetics and drug interactions. Cooperativity occurs when the binding of one ligand to a multi-subunit protein alters the affinity of other binding sites, enhancing or inhibiting overall activity, as observed in hemoglobin oxygen binding. Understanding these concepts is crucial for interpreting biochemical mechanisms, drug efficacy, and protein function modulation.
Fundamental Concepts in Molecular Interactions
Additivity in molecular interactions refers to the independent contributions of individual binding sites or forces, where the total effect equals the sum of each component's effect. Cooperativity occurs when binding at one site influences binding affinity at another, resulting in non-linear, amplified responses often seen in allosteric enzymes and multi-subunit proteins. Understanding the fundamental distinction between additive and cooperative interactions is essential in drug design, signal transduction, and enzyme regulation.
Mechanisms of Additive Effects
Additive effects occur when the combined impact of two or more agents equals the sum of their individual effects, often explained by independent binding or action at separate sites. Mechanisms of additive effects include multiple drugs targeting different receptors or pathways without influencing each other's activity, leading to predictable combined responses. This contrasts with cooperativity, where interactions between binding sites alter the overall effect beyond a simple sum.
Exploring Cooperative Interactions
Cooperative interactions occur when the binding of one molecule to a target enhances the affinity or activity of additional molecules, resulting in a non-linear response that differs from simple additivity. Unlike additive effects, where each ligand contributes independently to the overall effect, cooperativity involves conformational changes or allosteric mechanisms that amplify or suppress ligand binding. This phenomenon is critical in biological systems such as hemoglobin oxygen binding and enzyme regulation, where cooperative binding ensures precise control and sensitivity.
Mathematical Models: Additivity vs Cooperativity
Mathematical models distinguish additivity and cooperativity by their representation of interaction effects among molecules. Additivity models assume independent binding sites, resulting in linear combination of effects, often described by the sum of individual binding probabilities. Cooperativity models incorporate interaction terms and nonlinear dynamics, frequently using the Hill equation or Monod-Wyman-Changeux framework to capture enhanced or inhibited binding affinities dependent on ligand occupancy.
Biological Significance of Cooperativity
Cooperativity enhances biological regulation by allowing enzymes or receptors to respond more sensitively and dynamically to changes in ligand concentration, optimizing metabolic pathways and signal transduction. This phenomenon enables allosteric proteins, such as hemoglobin, to increase their binding affinity upon ligand interaction, facilitating efficient oxygen transport and delivery in tissues. By contrast, additivity describes independent binding events without such amplified responses, lacking the nuanced control critical for complex cellular functions.
Examples of Additive Interactions in Biology
Additive interactions in biology occur when the combined effect of two factors equals the sum of their individual effects, such as the combined action of two different antibiotics targeting separate bacterial processes. Vitamin A and zinc supplementation together improve immune function more effectively than either alone, demonstrating additive effects on health outcomes. In metabolic pathways, the contributions of multiple enzymes to substrate conversion often add up linearly, reflecting additive biochemical interactions.
Real-World Applications of Cooperativity
Cooperativity plays a crucial role in biochemical processes such as oxygen transport by hemoglobin, where binding of one oxygen molecule increases the affinity for subsequent molecules, enabling efficient oxygen delivery to tissues. In enzyme kinetics, cooperative binding allows for precise regulation of metabolic pathways, exemplified by allosteric enzymes like phosphofructokinase controlling glycolysis. Drug design leverages cooperativity to enhance therapeutic efficacy through molecules that modulate receptor activity more selectively than additive effects alone.
Experimental Approaches to Distinguish Additivity and Cooperativity
Experimental approaches to distinguish additivity from cooperativity often involve measuring binding affinities and reaction rates under varying ligand concentrations using techniques such as isothermal titration calorimetry (ITC) or surface plasmon resonance (SPR). Hill plots and binding curve analyses help quantify the degree of cooperativity by assessing deviations from the expected additive behavior of individual binding sites. Mutagenesis experiments combined with kinetic assays further clarify the cooperative interactions by isolating individual binding contributions and observing resultant functional changes.
Future Perspectives in Studying Additivity and Cooperativity
Future perspectives in studying additivity and cooperativity emphasize the integration of advanced computational modeling with high-throughput experimental techniques to unravel complex molecular interactions. Machine learning approaches are expected to enhance the prediction accuracy of cooperative effects in multi-component systems by analyzing vast datasets from protein-ligand binding and enzyme kinetics assays. Innovations in single-molecule spectroscopy and cryo-electron microscopy will provide unprecedented structural insights, enabling precise characterization of additive versus cooperative binding mechanisms at atomic resolution.
Additivity Infographic
 libterm.com
libterm.com