ELISA is a highly sensitive immunoassay technique used to detect and quantify specific proteins, antibodies, or hormones in biological samples. By harnessing antigen-antibody interactions and colorimetric detection, ELISA provides accurate diagnostics for various diseases and research applications. Explore the article to understand how ELISA can enhance your laboratory diagnostics and research outcomes.
Table of Comparison
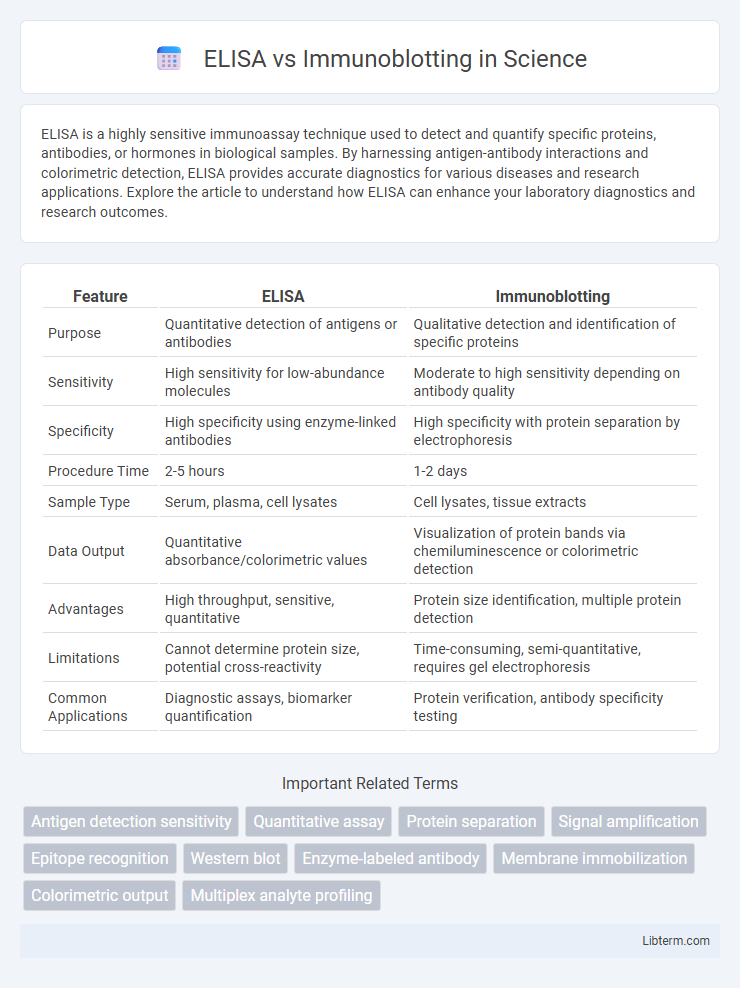
| Feature | ELISA | Immunoblotting |
|---|---|---|
| Purpose | Quantitative detection of antigens or antibodies | Qualitative detection and identification of specific proteins |
| Sensitivity | High sensitivity for low-abundance molecules | Moderate to high sensitivity depending on antibody quality |
| Specificity | High specificity using enzyme-linked antibodies | High specificity with protein separation by electrophoresis |
| Procedure Time | 2-5 hours | 1-2 days |
| Sample Type | Serum, plasma, cell lysates | Cell lysates, tissue extracts |
| Data Output | Quantitative absorbance/colorimetric values | Visualization of protein bands via chemiluminescence or colorimetric detection |
| Advantages | High throughput, sensitive, quantitative | Protein size identification, multiple protein detection |
| Limitations | Cannot determine protein size, potential cross-reactivity | Time-consuming, semi-quantitative, requires gel electrophoresis |
| Common Applications | Diagnostic assays, biomarker quantification | Protein verification, antibody specificity testing |
Introduction to ELISA and Immunoblotting
ELISA (Enzyme-Linked Immunosorbent Assay) is a highly sensitive technique used for detecting and quantifying specific proteins or antibodies in complex samples using antigen-antibody interactions and enzyme-mediated color change. Immunoblotting, also known as Western blotting, separates proteins by gel electrophoresis before transferring them onto a membrane for targeted antibody detection, enabling protein identification and size estimation. Both methods are fundamental in immunology and molecular biology for diagnostic and research applications focused on protein analysis.
Principle and Workflow of ELISA
ELISA (Enzyme-Linked Immunosorbent Assay) operates on the principle of antigen-antibody interaction, using enzyme-labeled antibodies to detect and quantify specific proteins in a sample through a colorimetric change. The workflow involves coating a microplate with antigen or antibody, blocking non-specific binding sites, adding samples, incubating with enzyme-linked detection antibodies, and then adding a substrate to produce a measurable color change. ELISA is widely used for its high sensitivity, specificity, and quantitative capabilities compared to immunoblotting, which relies on protein separation by gel electrophoresis and antibody detection on membranes.
Principle and Workflow of Immunoblotting
Immunoblotting, also known as Western blotting, is a technique that detects specific proteins through their separation by gel electrophoresis, transfer to a membrane, and subsequent antibody probing, enabling precise identification and quantification. The workflow involves protein extraction, SDS-PAGE gel electrophoresis to separate proteins by molecular weight, transfer onto a nitrocellulose or PVDF membrane, blocking to prevent non-specific binding, incubation with primary antibodies targeting the protein of interest, followed by secondary antibodies conjugated with enzymes or fluorophores for detection. This method provides high specificity and sensitivity for protein analysis, especially useful for confirming ELISA results and studying protein expression, modifications, or interactions.
Key Differences in Detection Mechanisms
ELISA utilizes enzyme-linked antibodies to produce a quantifiable colorimetric or fluorescent signal upon antigen-antibody binding, offering high sensitivity and scalability for detecting proteins or antibodies in samples. Immunoblotting, or Western blotting, separates proteins by gel electrophoresis before transferring them to membranes for antibody probing, enabling precise identification of protein size and post-translational modifications. ELISA provides quantitative results ideal for large sample sets, while immunoblotting offers qualitative and semi-quantitative insights with molecular weight specificity.
Sensitivity and Specificity Comparison
ELISA demonstrates high sensitivity through enzyme-mediated signal amplification, enabling detection of low-abundance antigens with minimal background noise. Immunoblotting offers superior specificity by separating proteins via electrophoresis before antibody probing, which reduces cross-reactivity and confirms molecular weight. While ELISA excels in quantifying targets in complex mixtures, immunoblotting provides detailed protein characterization essential for differentiating closely related epitopes.
Sample Preparation and Processing
ELISA requires minimal sample preparation with liquid samples often used directly after dilution, facilitating high-throughput processing. Immunoblotting demands more rigorous preparation, including protein extraction, quantification, and denaturation, followed by gel electrophoresis and membrane transfer. The detailed processing in immunoblotting provides protein size information, whereas ELISA emphasizes antigen concentration in native conditions.
Quantitative vs Qualitative Analysis
ELISA provides quantitative analysis by measuring antigen or antibody concentrations through colorimetric changes, enabling precise detection of specific proteins in complex samples. Immunoblotting, or Western blotting, offers qualitative and semi-quantitative information by identifying protein presence and molecular weight via antibody binding and band intensity visualization. While ELISA excels in high-throughput quantification, immunoblotting is preferred for confirming protein identity and assessing protein size variations.
Typical Applications in Research and Diagnostics
ELISA is extensively utilized for quantifying soluble antigens and antibodies in clinical diagnostics, such as hormone levels, infectious disease markers, and cytokine profiling. Immunoblotting excels in detecting specific proteins from complex mixtures, enabling identification of post-translational modifications and verification of protein expression in research fields like proteomics and autoimmune disease diagnosis. Both techniques complement each other, with ELISA preferred for high-throughput analysis and immunoblotting favored for qualitative and confirmatory assessments.
Advantages and Limitations of Each Technique
ELISA offers high sensitivity and quantification capabilities, making it ideal for detecting low concentrations of antigens or antibodies in large sample volumes, but it may suffer from cross-reactivity and limited specificity. Immunoblotting provides detailed information on protein size and post-translational modifications with greater specificity due to separation by electrophoresis, yet it is more time-consuming and less suitable for high-throughput analysis. ELISA's ease of automation and rapid processing contrasts with immunoblotting's ability to confirm protein identity and detect multiple protein isoforms simultaneously.
Choosing Between ELISA and Immunoblotting
Choosing between ELISA and immunoblotting depends on the specific diagnostic or research objective, as ELISA offers high-throughput quantification of antigen or antibody levels with excellent sensitivity and specificity. Immunoblotting provides detailed information about protein size, post-translational modifications, and antibody specificity by detecting proteins separated by gel electrophoresis. For large-scale screening, ELISA is preferred, while immunoblotting is ideal for confirming protein identity and analyzing complex protein mixtures.
ELISA Infographic
 libterm.com
libterm.com