Anabolic processes play a crucial role in muscle growth and tissue repair by promoting the synthesis of complex molecules from simpler ones. Understanding how anabolic hormones like testosterone and insulin function can significantly enhance your fitness and recovery strategies. Explore the full article to learn how optimizing anabolic pathways can transform your physical performance.
Table of Comparison
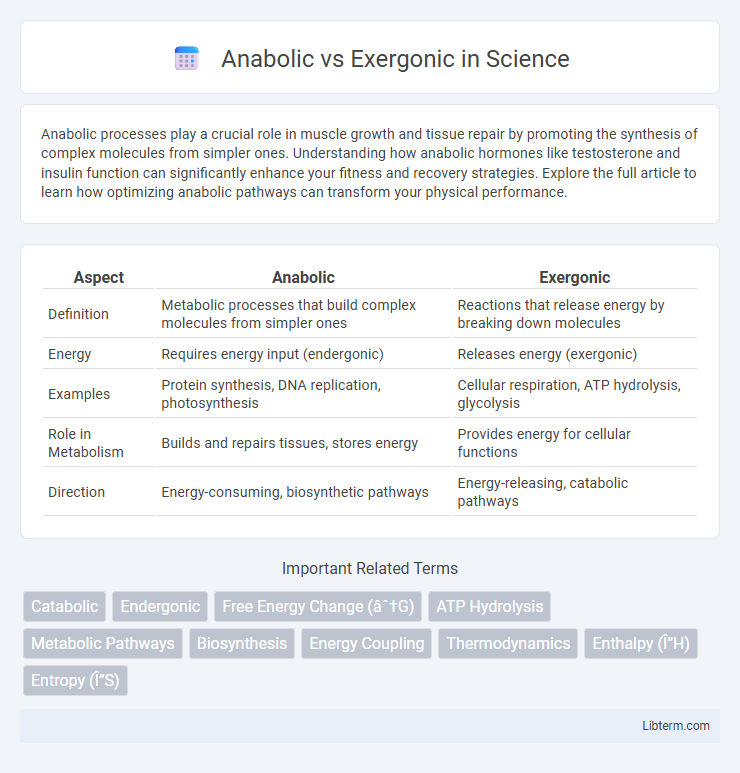
| Aspect | Anabolic | Exergonic |
|---|---|---|
| Definition | Metabolic processes that build complex molecules from simpler ones | Reactions that release energy by breaking down molecules |
| Energy | Requires energy input (endergonic) | Releases energy (exergonic) |
| Examples | Protein synthesis, DNA replication, photosynthesis | Cellular respiration, ATP hydrolysis, glycolysis |
| Role in Metabolism | Builds and repairs tissues, stores energy | Provides energy for cellular functions |
| Direction | Energy-consuming, biosynthetic pathways | Energy-releasing, catabolic pathways |
Introduction: Defining Anabolic and Exergonic
Anabolic processes involve the synthesis of complex molecules from simpler ones, requiring energy input to build cellular components such as proteins and nucleic acids. Exergonic reactions release energy by breaking down molecules during metabolic activities, facilitating energy transfer within cells. Understanding the roles of anabolic and exergonic reactions is essential for comprehending cellular metabolism and energy flow.
Core Concepts: Metabolism and Energy Transformation
Anabolic processes consume energy to build complex molecules from simpler ones, playing a crucial role in growth and repair within metabolism. Exergonic reactions release energy by breaking down molecules, driving cellular activities and maintaining metabolic balance. Together, these opposing energy transformations regulate the organism's energy flow essential for survival and function.
Anabolic Reactions: Building Complexity
Anabolic reactions involve the synthesis of complex molecules from simpler ones, requiring energy input to form chemical bonds. These processes are essential for cellular growth, repair, and storage of energy, prominently featuring pathways such as protein synthesis and nucleic acid formation. By consuming ATP and reducing equivalents like NADPH, anabolic reactions build molecular complexity critical for maintaining biological structure and function.
Exergonic Reactions: Releasing Chemical Energy
Exergonic reactions release chemical energy by breaking down complex molecules into simpler ones, resulting in a net release of free energy that can be harnessed for cellular processes. These reactions often involve the hydrolysis of ATP, where the high-energy phosphate bonds are cleaved, powering metabolic activities such as muscle contraction and active transport. The spontaneity and negative Gibbs free energy change characterize exergonic reactions, making them essential for driving energy-requiring endergonic processes in biological systems.
Key Differences: Anabolic vs Exergonic
Anabolic processes involve the synthesis of complex molecules from simpler ones, requiring an input of energy, making them endergonic reactions. Exergonic reactions release energy by breaking down complex molecules into simpler products, driving biological processes like cellular respiration. The key difference lies in energy flow: anabolic pathways consume energy to build structures, whereas exergonic pathways generate energy by degrading substrates.
Biological Examples of Anabolic Reactions
Anabolic reactions involve the synthesis of complex molecules from simpler ones, requiring energy input, as seen in the formation of proteins from amino acids during muscle growth. Photosynthesis in plants is another anabolic process where carbon dioxide and water are converted into glucose, storing energy in chemical bonds. DNA replication, essential for cell division, also exemplifies anabolic reactions by assembling nucleotides into a new DNA strand.
Biological Examples of Exergonic Reactions
Cellular respiration is a primary example of an exergonic reaction, where glucose molecules are broken down to release energy in the form of ATP. Another biological example includes the hydrolysis of ATP to ADP and inorganic phosphate, which drives many endergonic processes by releasing free energy. Enzymatic reactions within metabolic pathways often couple exergonic steps to facilitate biosynthetic activities efficiently.
Energetics: ATP’s Role in Both Processes
Anabolic pathways consume energy by using ATP to build complex molecules from simpler ones, driving biosynthesis and cellular growth. Exergonic reactions release energy by breaking down molecules, resulting in ATP generation through catabolic processes like cellular respiration. ATP acts as a crucial energy currency, coupling these opposing reactions to maintain cellular energetics and homeostasis.
Impacts on Cellular Function and Health
Anabolic processes consume energy to build complex molecules essential for cell growth, repair, and maintenance, directly supporting cellular function and overall health by promoting tissue regeneration and metabolic balance. Exergonic reactions release energy by breaking down molecules, providing the necessary ATP to power cellular activities such as muscle contraction, nerve impulse propagation, and active transport. The balance between anabolic and exergonic reactions is critical, as disruptions can lead to metabolic disorders, impaired cellular repair, and reduced physiological performance.
Conclusion: Integrating Anabolic and Exergonic Pathways
Integrating anabolic and exergonic pathways is essential for cellular metabolism, as exergonic reactions release energy that drives anabolic processes requiring energy input to build complex molecules. The coupling of these pathways ensures efficient energy transfer, maintaining cellular homeostasis and supporting growth and repair mechanisms. Understanding this integration highlights the interdependence between catabolic energy release and anabolic biosynthesis in biological systems.
Anabolic Infographic
 libterm.com
libterm.com