Chemiluminescence is the emission of light during a chemical reaction without the involvement of heat. This phenomenon is widely utilized in forensic science, medical diagnostics, and environmental monitoring due to its high sensitivity and specificity. Discover how understanding chemiluminescence can enhance your knowledge and applications by reading the full article.
Table of Comparison
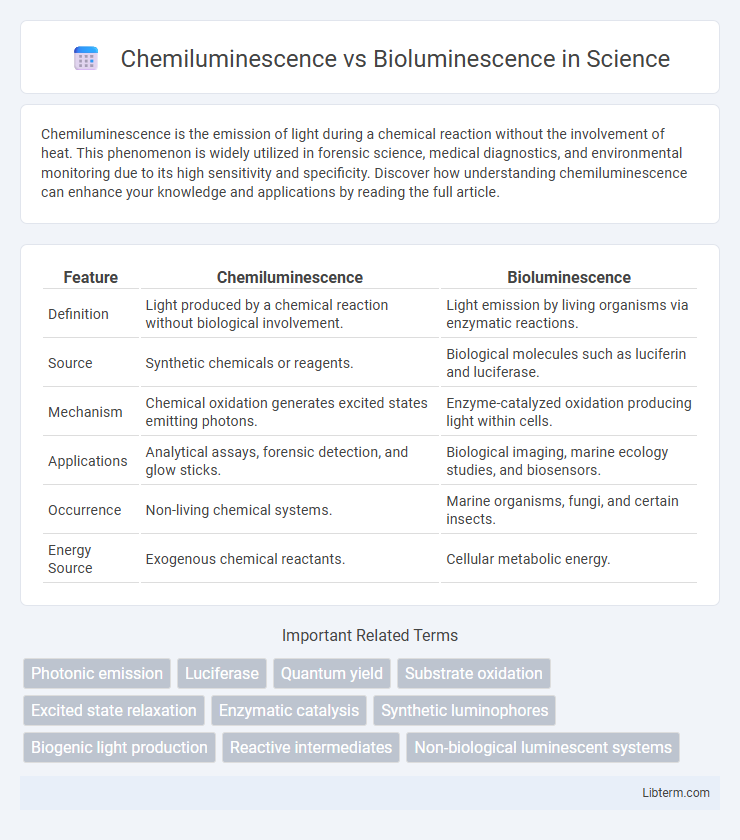
| Feature | Chemiluminescence | Bioluminescence |
|---|---|---|
| Definition | Light produced by a chemical reaction without biological involvement. | Light emission by living organisms via enzymatic reactions. |
| Source | Synthetic chemicals or reagents. | Biological molecules such as luciferin and luciferase. |
| Mechanism | Chemical oxidation generates excited states emitting photons. | Enzyme-catalyzed oxidation producing light within cells. |
| Applications | Analytical assays, forensic detection, and glow sticks. | Biological imaging, marine ecology studies, and biosensors. |
| Occurrence | Non-living chemical systems. | Marine organisms, fungi, and certain insects. |
| Energy Source | Exogenous chemical reactants. | Cellular metabolic energy. |
Introduction to Luminescence
Luminescence is the emission of light by a substance not resulting from heat, encompassing both chemiluminescence and bioluminescence. Chemiluminescence occurs when a chemical reaction produces an excited intermediate that emits photons as it returns to the ground state. In contrast, bioluminescence is a specific type of chemiluminescence found in living organisms, involving enzymes like luciferase that catalyze light-producing reactions.
Defining Chemiluminescence
Chemiluminescence is a chemical process in which light is emitted as a result of a chemical reaction, typically involving the oxidation of a substrate without the need for external light excitation. This phenomenon differs from bioluminescence, which is a specific type of chemiluminescence occurring naturally in living organisms through enzymatic reactions. Understanding chemiluminescence is essential for applications in analytical chemistry, forensic science, and medical diagnostics due to its high sensitivity and specificity.
Understanding Bioluminescence
Bioluminescence is a natural phenomenon where living organisms produce light through a chemical reaction involving luciferin, luciferase, oxygen, and ATP, contrasting with chemiluminescence, which occurs in non-living systems. This process is commonly observed in deep-sea creatures, fireflies, and certain fungi, serving ecological functions such as predation, camouflage, and communication. Understanding the molecular mechanisms of bioluminescence has advanced applications in biomedical imaging, environmental monitoring, and biosensors.
Chemical Reactions Behind the Glow
Chemiluminescence occurs when a chemical reaction produces an excited intermediate that releases energy as visible light upon returning to its ground state, commonly seen in luminol and oxalate ester reactions. Bioluminescence, a subset of chemiluminescence, specifically involves enzymatic reactions in living organisms where luciferase enzymes catalyze the oxidation of luciferin substrates, emitting light with high efficiency. The key difference lies in the biological context and enzymatic control in bioluminescence, versus the purely chemical or synthetic processes driving chemiluminescence.
Key Differences Between Chemiluminescence and Bioluminescence
Chemiluminescence is the emission of light resulting from a chemical reaction without the involvement of living organisms, while bioluminescence specifically refers to light produced by living organisms through enzymatic reactions involving luciferase enzymes. Bioluminescence typically serves biological functions such as communication, predation, or camouflage, whereas chemiluminescence is primarily utilized in analytical chemistry, medical diagnostics, and forensic applications. The molecular mechanisms differ as chemiluminescence involves non-biological catalysts, whereas bioluminescence requires biological substrates and enzyme-mediated pathways.
Natural vs Synthetic Light-Producing Systems
Chemiluminescence involves synthetic light production through chemical reactions without the involvement of living organisms, commonly used in laboratory assays and glow sticks. Bioluminescence is a natural phenomenon where living organisms, such as fireflies and certain marine animals, produce light via enzymatic reactions involving luciferase enzymes and luciferin substrates. These biological systems offer highly efficient, environmentally friendly light emission distinct from the purely chemical mechanisms of synthetic chemiluminescent devices.
Applications of Chemiluminescence in Science and Industry
Chemiluminescence finds widespread applications in science and industry, notably in forensic analysis where it aids in detecting trace amounts of blood through luminol-based reactions. It is crucial in environmental monitoring for measuring pollutants like hydrogen peroxide and ozone due to its high sensitivity and specificity. In clinical diagnostics, chemiluminescent assays provide rapid, accurate detection of biomarkers and pathogens, enhancing disease diagnosis and healthcare outcomes.
Bioluminescence in Nature: Examples and Functions
Bioluminescence in nature occurs when living organisms, such as fireflies, deep-sea fish, and certain fungi, produce light through enzymatic reactions involving luciferin and luciferase. This natural light emission serves vital functions including predator avoidance, prey attraction, and communication within species. Unlike chemiluminescence, which can be artificially triggered in laboratory settings, bioluminescence is a highly specialized biological process evolved to optimize survival strategies in various ecosystems.
Advantages and Limitations of Each Process
Chemiluminescence offers high sensitivity and low background interference, making it ideal for analytical assays but requires chemical reagents that can be unstable or hazardous. Bioluminescence provides natural light emission through enzyme-substrate reactions, enabling real-time biological imaging with minimal toxicity, though its light intensity is often lower and dependent on living organisms. Both processes excel in specific applications yet face challenges in stability, intensity, and environmental dependence.
Future Trends in Luminescence Research
Future trends in luminescence research emphasize the integration of chemiluminescence and bioluminescence for enhanced sensitivity in medical diagnostics and environmental monitoring. Advances in nanotechnology and synthetic biology are driving the development of highly efficient, tunable luminescent probes with applications in real-time imaging and point-of-care testing. Emerging materials and engineered enzymes promise to expand the versatility and sustainability of luminescent systems, enabling breakthroughs in both clinical and industrial fields.
Chemiluminescence Infographic
 libterm.com
libterm.com