Exothermic reactions release heat energy into the surroundings, often causing an increase in temperature. These processes play a crucial role in various natural and industrial applications, such as combustion and cellular respiration. Explore the article to understand how exothermic reactions impact your daily life and the environment.
Table of Comparison
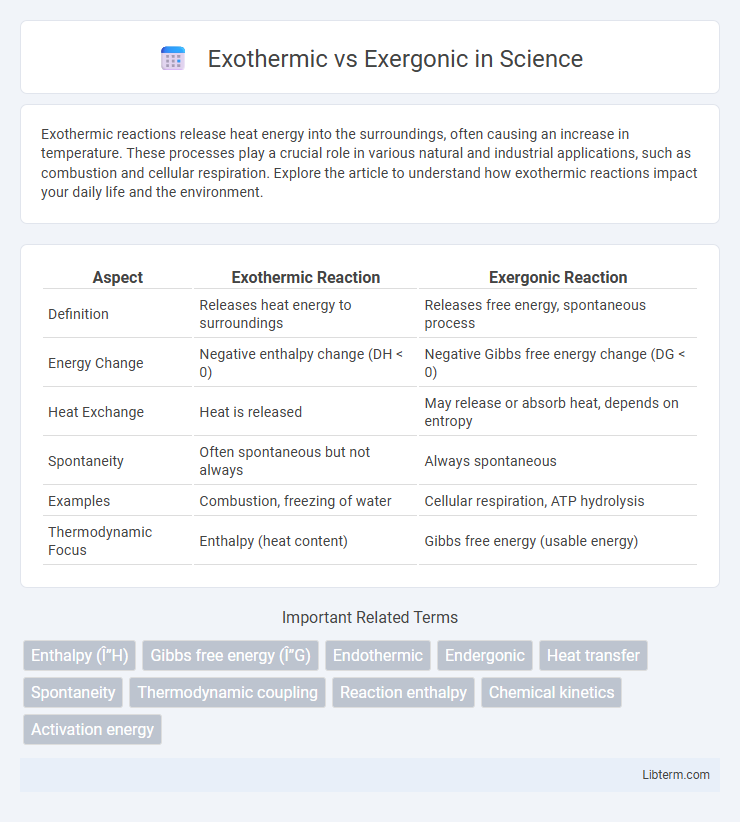
| Aspect | Exothermic Reaction | Exergonic Reaction |
|---|---|---|
| Definition | Releases heat energy to surroundings | Releases free energy, spontaneous process |
| Energy Change | Negative enthalpy change (DH < 0) | Negative Gibbs free energy change (DG < 0) |
| Heat Exchange | Heat is released | May release or absorb heat, depends on entropy |
| Spontaneity | Often spontaneous but not always | Always spontaneous |
| Examples | Combustion, freezing of water | Cellular respiration, ATP hydrolysis |
| Thermodynamic Focus | Enthalpy (heat content) | Gibbs free energy (usable energy) |
Understanding Exothermic and Exergonic Reactions
Exothermic reactions release heat energy into the surroundings, leading to a temperature increase, while exergonic reactions release free energy, indicating a spontaneous process with a negative Gibbs free energy change (DG < 0). Exothermic reactions are strictly concerned with the exchange of heat energy (enthalpy change, DH < 0), whereas exergonic reactions encompass broader energy changes, including both enthalpy and entropy contributions, driving metabolic and biochemical processes. Understanding the distinction clarifies that all exothermic reactions are not necessarily exergonic, as some may not be spontaneous under standard conditions.
Defining Exothermic Reactions
Exothermic reactions release heat energy to their surroundings, resulting in a temperature increase, as seen in combustion and respiration processes. These reactions have a negative enthalpy change (DH < 0) because the system loses energy in the form of heat. Exothermicity is a key factor in chemical thermodynamics, distinguishing it from exergonic reactions that focus on Gibbs free energy changes.
Defining Exergonic Reactions
Exergonic reactions are chemical processes that release free energy, resulting in a negative Gibbs free energy change (DG < 0), indicating spontaneity under constant temperature and pressure. These reactions drive cellular metabolism by coupling energy release to energy-requiring processes, crucial for ATP synthesis and biochemical pathways. Unlike exothermic reactions, which focus solely on heat release, exergonic reactions encompass overall energy changes, including enthalpy and entropy contributions.
Key Differences: Exothermic vs Exergonic
Exothermic reactions release heat energy to the surroundings, resulting in a negative enthalpy change (DH < 0), while exergonic reactions release free energy, characterized by a negative Gibbs free energy change (DG < 0). The key difference lies in energy type: exothermic processes specifically concern enthalpy and heat flow, whereas exergonic processes encompass overall spontaneity and thermodynamic favorability, including entropy effects. Understanding these distinctions is crucial in fields like chemistry and biochemistry for predicting reaction behavior and energy transformations.
Thermodynamics: Energy and Enthalpy Explained
Exothermic reactions release heat, indicating a negative enthalpy change (DH < 0), whereas exergonic reactions have a negative Gibbs free energy change (DG < 0), signifying spontaneous processes. Enthalpy (H) measures heat content, while Gibbs free energy integrates enthalpy, entropy, and temperature to determine reaction spontaneity. Understanding the distinction between energy flow (enthalpy change) and overall thermodynamic favorability (Gibbs free energy) is essential in chemical thermodynamics.
Real-World Examples of Exothermic Reactions
Exothermic reactions release heat energy, making combustion of gasoline and respiration in living organisms common real-world examples. The combustion of methane in natural gas stoves rapidly produces heat and light, demonstrating an exothermic process crucial for residential heating. Cellular respiration converts glucose and oxygen into carbon dioxide and water, releasing energy necessary for biological functions.
Real-World Examples of Exergonic Reactions
Exergonic reactions release free energy and occur spontaneously, making them essential in biological processes such as cellular respiration, where glucose is broken down to produce ATP. Another real-world example is the combustion of gasoline in engines, releasing energy that powers vehicles. Photosynthesis, although endergonic overall, includes exergonic steps where ATP is hydrolyzed, driving the synthesis of glucose.
Importance in Biology and Chemistry
Exothermic and exergonic reactions are crucial in biology and chemistry for understanding energy transformations in metabolic pathways and chemical processes. Exothermic reactions release heat, essential for maintaining body temperature and driving biochemical reactions, while exergonic reactions release free energy, enabling cellular activities like ATP synthesis and enzyme function. Distinguishing these terms aids in grasping reaction spontaneity and energy flow critical for life and industrial applications.
Applications in Industry and Everyday Life
Exothermic reactions, which release heat, are widely applied in industries such as metal forging, where the heat generated facilitates shaping and welding metals efficiently. Exergonic processes, involving the release of free energy, drive biochemical reactions essential for energy production in living organisms and biofuel development. Everyday applications include hand warmers using exothermic reactions and cellular respiration as an exergonic process powering human activity.
Summary: Choosing the Right Term
Exothermic reactions release heat energy to the surroundings, emphasizing thermal energy change, while exergonic reactions describe processes that release free energy, highlighting spontaneity and thermodynamic favorability. Selecting the right term depends on context: use exothermic for heat exchange in chemical reactions and exergonic when assessing Gibbs free energy and reaction spontaneity. Accurate terminology ensures clear communication in thermodynamics and chemical kinetics studies.
Exothermic Infographic
 libterm.com
libterm.com