Meso compounds are unique stereoisomers with multiple chiral centers that are superimposable on their mirror images due to an internal plane of symmetry, resulting in optical inactivity. Understanding the properties and behavior of meso compounds is crucial in stereochemistry and influences reactions and mechanisms in organic chemistry. Explore the rest of the article to deepen your knowledge of meso compounds and their significance in chemical synthesis.
Table of Comparison
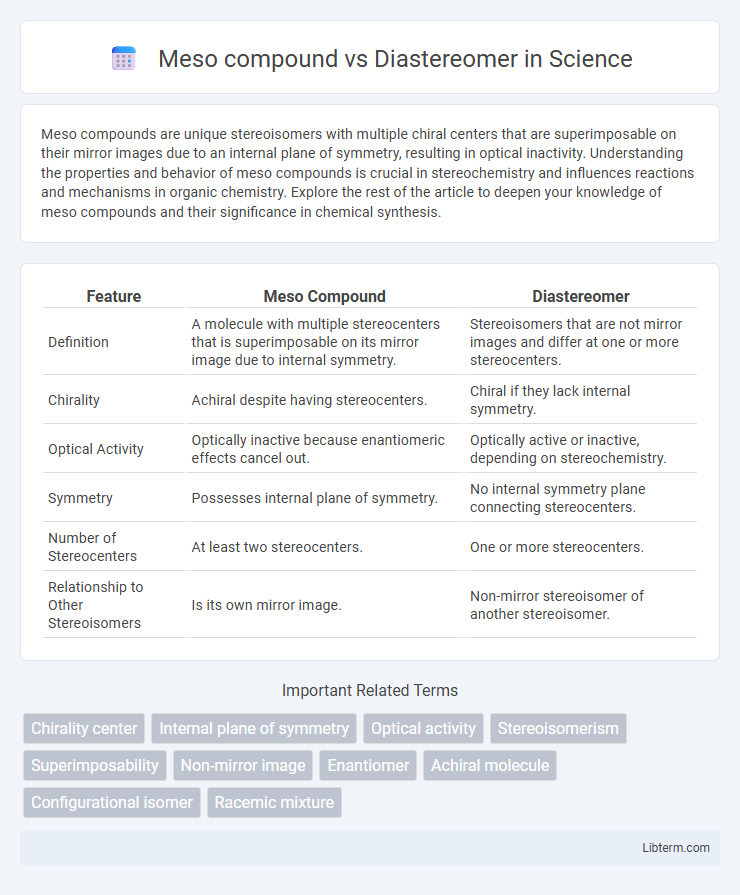
| Feature | Meso Compound | Diastereomer |
|---|---|---|
| Definition | A molecule with multiple stereocenters that is superimposable on its mirror image due to internal symmetry. | Stereoisomers that are not mirror images and differ at one or more stereocenters. |
| Chirality | Achiral despite having stereocenters. | Chiral if they lack internal symmetry. |
| Optical Activity | Optically inactive because enantiomeric effects cancel out. | Optically active or inactive, depending on stereochemistry. |
| Symmetry | Possesses internal plane of symmetry. | No internal symmetry plane connecting stereocenters. |
| Number of Stereocenters | At least two stereocenters. | One or more stereocenters. |
| Relationship to Other Stereoisomers | Is its own mirror image. | Non-mirror stereoisomer of another stereoisomer. |
Introduction to Stereoisomerism
Stereoisomerism involves molecules with the same molecular formula and connectivity but differing spatial arrangements. Meso compounds are achiral molecules containing multiple stereocenters with an internal plane of symmetry, resulting in optical inactivity despite stereocenters. Diastereomers are stereoisomers that are not mirror images, possessing different physical and chemical properties, and include configurations that are neither enantiomers nor meso compounds.
Defining Meso Compounds
Meso compounds are achiral molecules containing multiple stereocenters with an internal plane of symmetry, resulting in optical inactivity despite having chiral centers. Diastereomers are stereoisomers that are not mirror images and possess different chemical and physical properties. Unlike diastereomers, meso compounds are unique due to their internal symmetry and lack of enantiomeric pairs.
Understanding Diastereomers
Diastereomers are stereoisomers that are not mirror images of each other and differ in the spatial arrangement of atoms at one or more stereogenic centers, unlike meso compounds which contain internal symmetry making them achiral despite multiple stereocenters. The key distinction lies in diastereomers exhibiting different physical and chemical properties, such as melting points and reactivity, whereas meso compounds do not have optical activity due to their symmetry. Understanding diastereomers is essential in stereochemistry for distinguishing compounds that influence biological activity and pharmaceutical efficacy.
Key Structural Features of Meso Compounds
Meso compounds exhibit internal symmetry and contain multiple stereocenters, resulting in overall optical inactivity despite the presence of chiral centers. Their key structural feature is a plane of symmetry that divides the molecule into two mirror-image halves, allowing superimposability on their mirror image. Diastereomers differ as they are stereoisomers that are not mirror images and lack such internal symmetry, leading to distinct physical and chemical properties.
Characteristics of Diastereomers
Diastereomers are stereoisomers that are not mirror images and typically exhibit different physical and chemical properties, such as melting points, boiling points, and reactivity, unlike meso compounds which are achiral despite having stereocenters. Diastereomers can have multiple stereocenters with at least one differing configuration, resulting in distinct spatial arrangements that affect molecular interactions. This difference in three-dimensional structure often leads to varied biological activities and crystallization behaviors, making diastereomers important in pharmaceutical and chemical synthesis contexts.
Symmetry and Chirality: Core Differences
Meso compounds exhibit internal plane symmetry, rendering them achiral despite having multiple stereocenters, which distinguishes them from diastereomers that lack such symmetry and are inherently chiral. The presence of an internal mirror plane in meso compounds cancels optical activity, whereas diastereomers display different physical and chemical properties due to their distinct spatial arrangements. Understanding these core differences in symmetry and chirality is essential for stereochemical analysis and predicting molecular behavior in chemical reactions.
Physical and Chemical Properties Comparison
Meso compounds exhibit identical physical properties such as melting points and solubility to their enantiomers due to internal symmetry and achirality, whereas diastereomers differ significantly in physical properties including melting points, boiling points, and refractive indices. Chemically, meso compounds show distinct reactivity patterns since they are achiral and do not exhibit optical activity, unlike diastereomers which often have different stereochemistry leading to variable chemical reactivity and optical rotation. The differences in stereochemistry between meso compounds and diastereomers directly influence their physical characteristics and chemical behavior, crucial in stereoselective synthesis and chiral resolution processes.
Methods to Identify Meso Compounds and Diastereomers
Meso compounds can be identified by locating an internal plane of symmetry within the molecule, which results in optical inactivity despite multiple stereocenters, and confirming this by measuring zero optical rotation with a polarimeter. Diastereomers are differentiated through their distinct physical and chemical properties, such as melting points, boiling points, and NMR spectral data, which do not mirror each other like enantiomers. Chromatographic techniques and chiral resolution methods further assist in distinguishing diastereomers from meso compounds based on their differing interactions with chiral environments.
Real-World Examples and Applications
Meso compounds, such as tartaric acid, exhibit optical inactivity despite having chiral centers due to their internal plane of symmetry, making them crucial in stereochemistry studies and pharmaceuticals for enantiomer separation. Diastereomers, exemplified by cis- and trans-2-butene or various stereoisomers of glucose, differ in physical properties and reactivity, important in drug design and synthesis where selective biological activity is desired. Understanding these differences enables chemists to manipulate stereochemistry for improved efficacy and reduced side effects in medicinal chemistry.
Summary: Meso Compounds vs. Diastereomers
Meso compounds are achiral molecules containing multiple stereocenters with an internal plane of symmetry, causing them to be superimposable on their mirror images. Diastereomers are stereoisomers with two or more stereocenters that are not mirror images and differ in physical and chemical properties. Unlike meso compounds, diastereomers exhibit chirality and lack internal symmetry.
Meso compound Infographic
 libterm.com
libterm.com