Reversible garments offer versatile styling options, allowing you to switch between looks effortlessly and maximize wardrobe functionality. These items are designed with durability and dual aesthetics, providing great value and convenience for those seeking efficient fashion choices. Explore the benefits of reversible clothing and how it can transform your daily outfit decisions in the rest of this article.
Table of Comparison
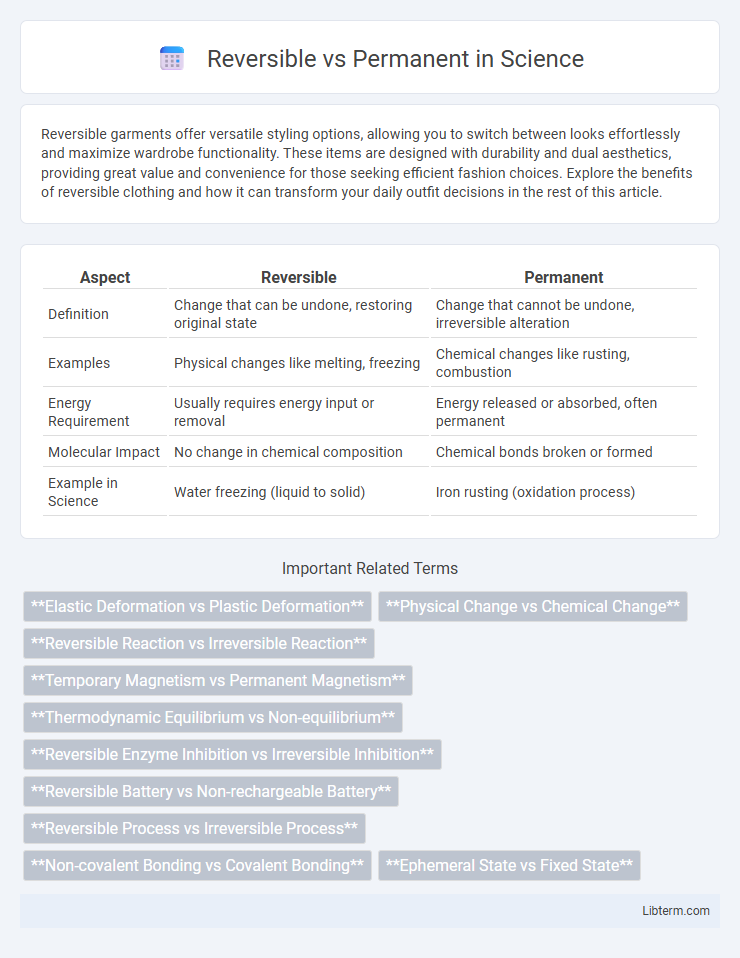
| Aspect | Reversible | Permanent |
|---|---|---|
| Definition | Change that can be undone, restoring original state | Change that cannot be undone, irreversible alteration |
| Examples | Physical changes like melting, freezing | Chemical changes like rusting, combustion |
| Energy Requirement | Usually requires energy input or removal | Energy released or absorbed, often permanent |
| Molecular Impact | No change in chemical composition | Chemical bonds broken or formed |
| Example in Science | Water freezing (liquid to solid) | Iron rusting (oxidation process) |
Understanding Reversible and Permanent Changes
Reversible changes involve modifications that can be undone, restoring the original state of a substance or object, such as melting ice reverting to water. Permanent changes alter the material or object's structure irreversibly, like rust forming on iron or wood burning. Understanding the distinction between reversible and permanent changes is essential in fields such as chemistry, material science, and environmental studies for predicting outcomes and managing processes effectively.
Key Differences: Reversible vs Permanent
Reversible processes allow a system to return to its original state without any net change in entropy, often occurring quasi-statically and maintaining equilibrium throughout. Permanent processes lead to irreversible changes, increasing entropy and preventing the system from restoring its initial conditions without external intervention. The key difference lies in entropy production: zero for reversible and positive for permanent processes.
Scientific Principles Behind Each Type
Reversible processes in thermodynamics involve changes that can be undone without increasing entropy, relying on gradual adjustments near equilibrium states to ensure no net loss of energy. Permanent changes, or irreversible processes, increase the system's entropy due to dissipative effects such as friction, turbulence, and spontaneous chemical reactions that prevent the system from returning to its original state. The fundamental scientific principle distinguishing the two is the second law of thermodynamics, which states that entropy in an isolated system never decreases, making permanent changes fundamentally entropic and irreversible.
Everyday Examples of Reversible Changes
Everyday examples of reversible changes include the melting of ice into water, boiling of water into steam, and dissolving sugar in tea, all of which can return to their original states without altering their chemical composition. Reversible changes demonstrate physical transformations where no new substance is formed, such as freezing water back into ice or evaporating water to condense again. These examples illustrate the distinction from permanent changes, where irreversible chemical reactions occur, like burning wood or cooking an egg.
Common Instances of Permanent Changes
Permanent changes often include tattoos, surgical alterations, and amputations, which cause irreversible modifications to the body. These instances result in lasting physical transformations that cannot be undone without significant intervention or are completely irreversible. Unlike reversible changes such as clothing or hairstyle, permanent changes fundamentally alter the original state.
Factors Influencing Reversibility
Factors influencing reversibility include the extent of tissue damage, duration of the insult, and the type of cells involved. Mild ischemia often leads to reversible injury due to temporary metabolic disturbances, while prolonged ischemia causes irreversible necrosis. Cellular resilience and timely restoration of blood flow significantly determine whether tissue recovers or undergoes permanent damage.
Advantages of Reversible Processes
Reversible processes maximize energy efficiency by minimizing entropy generation, thus maintaining thermodynamic equilibrium throughout the operation. These processes allow complete recovery of work and heat, leading to optimal system performance and reduced energy losses. Their ability to be reversed without net changes to the surroundings makes them ideal for designing sustainable and cost-effective thermodynamic cycles.
Implications of Permanent Transformations
Permanent transformations result in irreversible changes that fundamentally alter the original material or system, impacting its future usability and functionality. These irreversible modifications often require significant resources or replacement to restore original conditions, posing challenges in sustainability and cost-efficiency. Understanding the long-term consequences of permanent changes is crucial for industries prioritizing durability and environmental responsibility.
Environmental Impact: Reversible vs Permanent
Reversible environmental impacts allow ecosystems to recover naturally over time, preserving biodiversity and maintaining ecological balance. Permanent environmental impacts result in irreversible changes such as habitat destruction, loss of species, and long-term pollution that can disrupt ecosystem services. Prioritizing reversible practices mitigates long-lasting damage and supports sustainable resource management for future generations.
Choosing Between Reversible and Permanent Solutions
Choosing between reversible and permanent solutions depends on the context and desired outcomes. Reversible solutions, such as temporary adhesives or modular furniture, offer flexibility and easy adjustments, ideal for evolving needs or short-term use. Permanent solutions, like welded joints or fixed installations, provide durability and long-term stability but require careful planning to avoid costly modifications.
Reversible Infographic
 libterm.com
libterm.com