Coenzymes are organic molecules that bind to enzymes, enhancing their ability to catalyze biochemical reactions essential for cellular function and metabolism. These molecules assist in substrate conversion by temporarily attaching to the enzyme's active site, often acting as carriers of electrons or chemical groups. Discover how understanding coenzymes can improve your knowledge of metabolic processes in the full article.
Table of Comparison
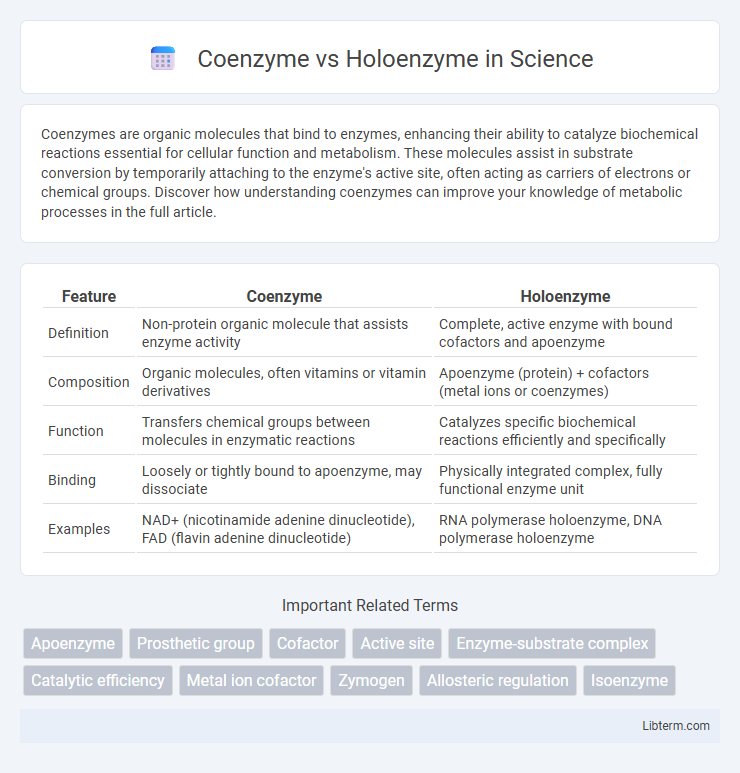
| Feature | Coenzyme | Holoenzyme |
|---|---|---|
| Definition | Non-protein organic molecule that assists enzyme activity | Complete, active enzyme with bound cofactors and apoenzyme |
| Composition | Organic molecules, often vitamins or vitamin derivatives | Apoenzyme (protein) + cofactors (metal ions or coenzymes) |
| Function | Transfers chemical groups between molecules in enzymatic reactions | Catalyzes specific biochemical reactions efficiently and specifically |
| Binding | Loosely or tightly bound to apoenzyme, may dissociate | Physically integrated complex, fully functional enzyme unit |
| Examples | NAD+ (nicotinamide adenine dinucleotide), FAD (flavin adenine dinucleotide) | RNA polymerase holoenzyme, DNA polymerase holoenzyme |
Introduction to Enzymes
Enzymes are biological catalysts that accelerate biochemical reactions by lowering activation energy and often require specific non-protein components to function properly. Coenzymes are organic molecules that bind temporarily to an enzyme to assist in the catalytic process, whereas holoenzymes refer to the complete, active enzyme complex consisting of the apoenzyme (protein portion) and its cofactor(s), including coenzymes or metal ions. Understanding the distinction between coenzyme and holoenzyme is essential in biochemical studies, as it highlights the role of auxiliary molecules in enzyme activity and overall metabolic regulation.
Defining Coenzymes
Coenzymes are organic non-protein molecules that bind temporarily to an enzyme's active site, assisting in catalysis by transferring chemical groups or electrons. They differ from holoenzymes, which are complete and active enzyme complexes consisting of an apoenzyme (protein portion) and its coenzyme or cofactor. Essential coenzymes such as NAD+, FAD, and coenzyme A play critical roles in metabolic pathways by facilitating enzymatic reactions.
What Are Holoenzymes?
Holoenzymes are fully functional enzyme complexes comprising an apoenzyme (protein portion) combined with its necessary cofactor or coenzyme, enabling catalytic activity. These cofactors can be organic molecules like vitamins (e.g., NAD+, FAD) or metal ions (e.g., Mg2+, Zn2+) that assist in the enzyme's biochemical reactions. Without the cofactor, the enzyme remains an inactive apoenzyme, making the holoenzyme essential for proper metabolic processes.
Structural Differences Between Coenzymes and Holoenzymes
Coenzymes are non-protein organic molecules that bind temporarily to an enzyme's active site, aiding in catalytic activity by transferring chemical groups or electrons. Holoenzymes consist of an apoenzyme (the protein portion) combined with its cofactor, which can be a coenzyme or metal ion, forming the active enzyme complex. The key structural difference lies in the holoenzyme's complete assembly, where the coenzyme is firmly integrated into the enzyme structure, enabling precise catalytic function.
Functional Roles in Biochemical Reactions
Coenzymes serve as non-protein organic molecules that bind temporarily to enzymes, facilitating the transfer of chemical groups during biochemical reactions and enhancing enzyme specificity and catalytic efficiency. Holoenzymes represent the complete, active enzyme form comprising both the apoenzyme (protein portion) and its bound cofactor or coenzyme, essential for biological activity and substrate conversion. The functional role of coenzymes lies in their role as carriers of electrons, atoms, or functional groups, whereas holoenzymes are crucial as fully assembled catalytic units enabling precise and efficient biochemical transformations.
Sources and Types of Coenzymes
Coenzymes are organic molecules that assist enzymes by carrying chemical groups between molecules, commonly derived from vitamins such as B-complex vitamins including niacin (NAD+/NADP+) and riboflavin (FAD). They differ from holoenzymes, which are complete enzymatic units comprising apoenzymes (protein portion) and their coenzyme or cofactor components. Types of coenzymes include NAD+/NADP+, FAD, and coenzyme A, each playing critical roles in metabolic pathways like oxidation-reduction and acyl group transfer.
Holoenzyme Activation and Assembly
Holoenzyme activation involves the precise assembly of an apoenzyme with its required cofactor, which can be a coenzyme or metal ion, enabling the enzyme to become catalytically active. This assembly ensures proper structural conformation for substrate binding and catalytic function, essential in processes such as DNA replication where DNA polymerase holoenzyme forms by combining core polymerase with accessory proteins. Efficient holoenzyme activation and assembly are critical for metabolic regulation, optimizing enzyme efficiency and specificity in biochemical pathways.
Importance in Metabolism and Health
Coenzymes serve as essential organic molecules that assist enzymes in catalyzing metabolic reactions, directly affecting energy production and nutrient utilization. Holoenzymes, the complete active enzyme complexes including their cofactors or coenzymes, are crucial for maintaining metabolic homeostasis and supporting vital physiological functions. Deficiencies or malfunctions in coenzymes or holoenzymes can disrupt metabolic pathways, leading to metabolic disorders and compromised overall health.
Key Examples: Coenzyme vs Holoenzyme in Action
Coenzymes function as organic non-protein molecules like NAD+ and FAD that bind temporarily to enzymes, assisting catalytic activity during metabolic reactions such as oxidation-reduction. Holoenzymes comprise the complete, active enzyme form including the apoenzyme and its cofactor or coenzyme; for instance, DNA polymerase holoenzyme contains multiple subunits essential for DNA replication. The distinction lies in coenzymes acting as helper molecules, whereas holoenzymes represent the fully functional enzyme complex necessary for substrate transformation.
Summary: Coenzymes vs Holoenzymes
Coenzymes are organic non-protein molecules that bind to enzymes temporarily, assisting in catalysis by transferring chemical groups or electrons. Holoenzymes refer to the complete, active enzyme complex consisting of the apoenzyme (protein portion) combined with its cofactor, which can be a coenzyme or metal ion. Unlike coenzymes, holoenzymes represent the fully functional form of the enzyme required for catalytic activity.
Coenzyme Infographic
 libterm.com
libterm.com