A prosthetic group is a non-protein molecule tightly bound to a protein, essential for its biological activity and function. These groups participate in crucial processes like enzyme catalysis, electron transfer, and stabilization of protein structure. Discover how prosthetic groups influence protein behavior and their vital roles by reading the rest of the article.
Table of Comparison
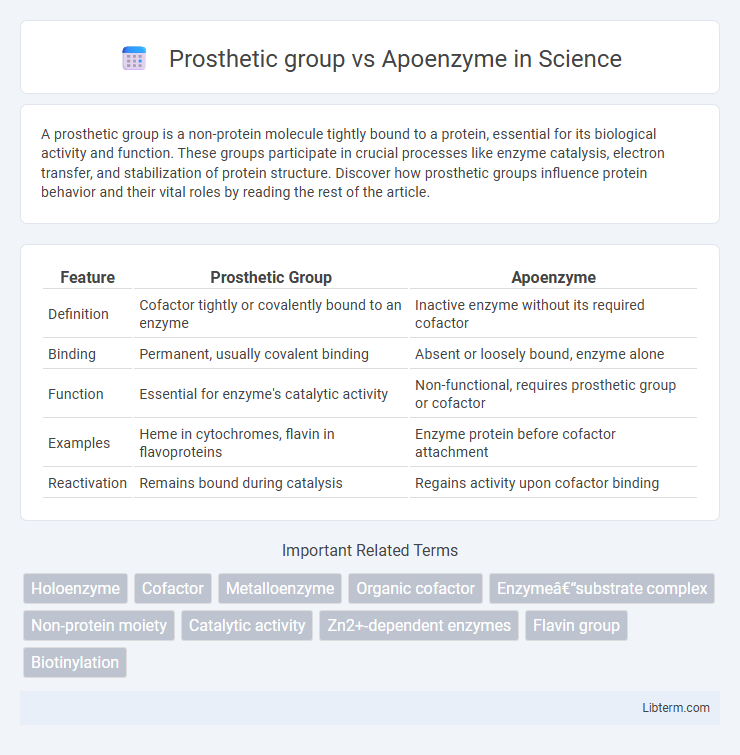
| Feature | Prosthetic Group | Apoenzyme |
|---|---|---|
| Definition | Cofactor tightly or covalently bound to an enzyme | Inactive enzyme without its required cofactor |
| Binding | Permanent, usually covalent binding | Absent or loosely bound, enzyme alone |
| Function | Essential for enzyme's catalytic activity | Non-functional, requires prosthetic group or cofactor |
| Examples | Heme in cytochromes, flavin in flavoproteins | Enzyme protein before cofactor attachment |
| Reactivation | Remains bound during catalysis | Regains activity upon cofactor binding |
Introduction to Prosthetic Groups and Apoenzymes
Prosthetic groups are tightly bound non-protein molecules essential for the biological activity of certain enzymes, serving as permanent cofactors that facilitate catalytic functions. Apoenzymes refer to the inactive protein portion of enzymes that require binding with a prosthetic group or cofactor to form a functional holoenzyme. This interplay between apoenzymes and prosthetic groups is critical in biochemical reactions, enabling enzymes to achieve their full catalytic potential.
Defining Prosthetic Groups
Prosthetic groups are tightly bound non-protein molecules essential for the biological activity of certain enzymes, often covalently attached to the enzyme's structure. They differ from apoenzymes, which are the inactive protein portions of enzymes lacking their necessary cofactor or prosthetic group. The presence of a prosthetic group transforms an apoenzyme into a holoenzyme, enabling catalytic functionality.
Understanding Apoenzymes
Apoenzymes are inactive enzyme proteins that require a prosthetic group or cofactor to become catalytically active; they lack the non-protein component necessary for their function. The prosthetic group is covalently bound to the apoenzyme, enabling the enzyme's proper structural configuration and catalytic activity. Understanding apoenzymes involves recognizing their dependence on these essential prosthetic groups to achieve the holoenzyme form, which performs specific biochemical reactions.
Structural Differences between Prosthetic Groups and Apoenzymes
Prosthetic groups are tightly bound non-protein molecules attached to enzymes, providing essential cofactors that stabilize the enzyme's active site and enable catalysis. Apoenzymes represent the protein portion of an enzyme without its cofactor, rendering the enzyme inactive until the prosthetic group or coenzyme is bound. Structurally, prosthetic groups integrate firmly with the apoenzyme's tertiary or quaternary structure, often through covalent or strong non-covalent bonds, while apoenzymes lack these non-protein components, leading to differences in enzyme conformation and functionality.
Functional Roles in Enzyme Activity
Prosthetic groups are non-protein molecules tightly bound to enzymes, essential for their catalytic activity by facilitating substrate binding or redox reactions. Apoenzymes are inactive enzyme protein components that require prosthetic groups or coenzymes to convert into active holoenzymes. The functional role of prosthetic groups is to enable enzymatic reactions by participating directly in the catalytic mechanism or stabilizing substrate-enzyme interactions, while apoenzymes provide the structural framework for proper enzyme conformation.
Types of Prosthetic Groups Commonly Found in Enzymes
Prosthetic groups are non-protein cofactors tightly bound to enzymes, essential for their catalytic activity and include types such as metal ions (e.g., Fe2+, Mg2+) and organic molecules like flavin adenine dinucleotide (FAD), heme, and biotin. Apoenzymes are the inactive protein portions of enzymes that require binding to their specific prosthetic group to become active holoenzymes. Common prosthetic groups involved in redox reactions, electron transfer, and carboxylation highlight their critical roles in enzyme function and metabolic pathways.
Mechanisms of Enzyme Activation
Prosthetic groups enable enzyme activation by tightly binding to the enzyme, facilitating the correct active site conformation necessary for catalytic activity. Apoenzymes, the inactive protein portions, require prosthetic groups or cofactors to form holoenzymes, the catalytically active complexes. This binding induces structural changes that stabilize the transition state and enhance substrate affinity, driving enzymatic reactions efficiently.
Examples of Enzymes with Prosthetic Groups versus Apoenzymes
Hemoglobin contains heme as a prosthetic group essential for oxygen transport, whereas carbonic anhydrase functions as an apoenzyme requiring zinc ions that act as a cofactor but are not tightly bound as prosthetic groups. Another example is flavoproteins, such as succinate dehydrogenase, which have flavin adenine dinucleotide (FAD) as a prosthetic group covalently attached. Lysozyme, in contrast, acts without a prosthetic group and functions as an apoenzyme that relies solely on its polypeptide structure for catalytic activity.
Biological Significance and Applications
Prosthetic groups are non-protein cofactors tightly bound to enzymes, essential for catalytic activity by facilitating electron transfer, substrate binding, or structural stability, whereas apoenzymes refer to the protein portion of an enzyme lacking its prosthetic group or cofactor and are biologically inactive until combined with the necessary non-protein component. The biological significance of prosthetic groups lies in their role in enabling key biochemical reactions, such as heme in cytochromes for electron transport and flavin in oxidoreductases, while apoenzymes provide a regulatory checkpoint controlling enzyme activation. Applications include drug design targeting prosthetic group binding sites to modulate enzyme activity and biotechnological enzyme engineering to enhance stability or specificity by manipulating prosthetic group interactions.
Summary: Key Differences and Implications
Prosthetic groups are tightly bound non-protein molecules essential for enzyme functionality, whereas apoenzymes are the inactive protein components of enzymes lacking their necessary cofactors. The presence of a prosthetic group converts an apoenzyme into a holoenzyme, enabling catalytic activity and substrate binding. Understanding this distinction is critical for biochemistry applications such as drug design and enzyme engineering.
Prosthetic group Infographic
 libterm.com
libterm.com