Chimeric molecules combine genetic material from different sources to create novel proteins with unique functions. These constructs are widely used in biotechnology and medical research to develop targeted therapies and diagnostic tools. Discover how chimeric technology could revolutionize your approach to science and medicine by exploring the rest of this article.
Table of Comparison
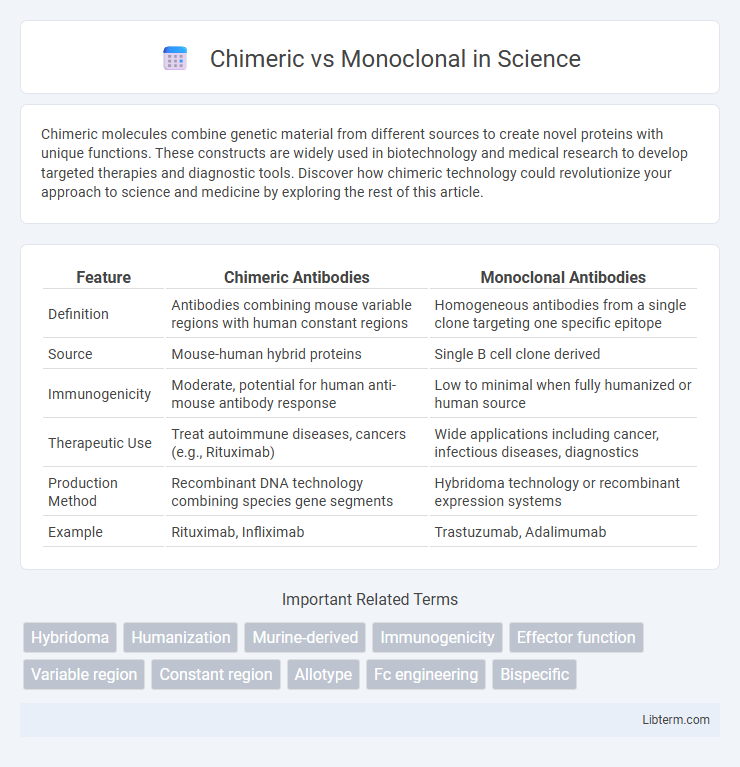
| Feature | Chimeric Antibodies | Monoclonal Antibodies |
|---|---|---|
| Definition | Antibodies combining mouse variable regions with human constant regions | Homogeneous antibodies from a single clone targeting one specific epitope |
| Source | Mouse-human hybrid proteins | Single B cell clone derived |
| Immunogenicity | Moderate, potential for human anti-mouse antibody response | Low to minimal when fully humanized or human source |
| Therapeutic Use | Treat autoimmune diseases, cancers (e.g., Rituximab) | Wide applications including cancer, infectious diseases, diagnostics |
| Production Method | Recombinant DNA technology combining species gene segments | Hybridoma technology or recombinant expression systems |
| Example | Rituximab, Infliximab | Trastuzumab, Adalimumab |
Introduction to Chimeric and Monoclonal Antibodies
Chimeric antibodies combine murine variable regions with human constant regions, enhancing immune compatibility and reducing immunogenicity, while monoclonal antibodies are identical immunoglobulins produced from a single clone of B cells, designed to target specific antigens with high precision. Chimeric antibodies typically exhibit around 65-70% human protein sequences, improving therapeutic efficacy in human patients compared to fully murine antibodies. Monoclonal antibodies, whether chimeric, humanized, or fully human, are widely used in targeted therapies for cancer, autoimmune diseases, and infectious diseases due to their specificity and consistent batch-to-batch production.
Basic Structure and Composition Differences
Chimeric antibodies consist of variable regions derived from one species, typically mouse, fused with constant regions from another, usually human, resulting in approximately 65-70% human protein content to reduce immunogenicity. Monoclonal antibodies are fully human or humanized, with identical heavy and light chains produced from a single clone, ensuring uniform specificity and minimal foreign protein sequences. The structural distinction lies in the variable domain origin and percentage of human sequence, influencing antibody stability, efficacy, and immune response.
Development Process of Chimeric Antibodies
The development process of chimeric antibodies involves combining the murine variable regions with human constant regions to reduce immunogenicity while preserving antigen specificity. This genetic engineering approach typically employs recombinant DNA technology and hybridoma cells to produce antibodies with enhanced compatibility for human therapeutic use. The resulting chimeric antibodies exhibit improved efficacy and safety profiles compared to fully murine antibodies in clinical applications.
Development Process of Monoclonal Antibodies
The development process of monoclonal antibodies involves isolating a specific B-cell that produces the desired antibody and fusing it with a myeloma cell to create a hybridoma, which can be cultured indefinitely for consistent antibody production. This method ensures high specificity and uniformity of monoclonal antibodies compared to chimeric antibodies, which combine murine and human antibody components through genetic engineering techniques. Monoclonal antibody development requires extensive screening and validation to confirm affinity, specificity, and functional activity before clinical application.
Mechanisms of Action and Target Specificity
Chimeric antibodies combine murine variable regions with human constant regions, enabling enhanced immune activation through Fc receptor binding while maintaining antigen specificity dictated by the murine variable domains. Monoclonal antibodies, fully human or humanized, exhibit precise target specificity by recognizing a single epitope, leading to mechanisms such as neutralization, antibody-dependent cellular cytotoxicity (ADCC), or complement-dependent cytotoxicity (CDC). The chimeric design may elicit immunogenicity due to murine components, affecting therapeutic efficacy, whereas monoclonal antibodies reduce immunogenic risk while optimizing target binding and immune effector functions.
Clinical Applications and Therapeutic Uses
Chimeric antibodies combine murine variable regions with human constant regions, enhancing specificity while reducing immunogenicity, and are widely used in oncology for targeted cancer therapies such as rituximab in non-Hodgkin lymphoma. Monoclonal antibodies, derived from a single clone of B cells, offer high specificity and are employed in treating autoimmune diseases, infectious diseases, and various cancers, exemplified by trastuzumab for HER2-positive breast cancer. Both chimeric and monoclonal antibodies serve critical roles in immunotherapy, with ongoing clinical trials expanding their applications across diverse therapeutic areas.
Efficacy and Safety Profiles
Chimeric antibodies combine murine and human components to enhance efficacy by improving immune response while maintaining acceptable safety profiles with moderate immunogenicity. Monoclonal antibodies, fully human or humanized, demonstrate superior safety due to reduced immunogenic reactions and maintain high efficacy by specifically targeting antigens. Clinical data indicates monoclonal antibodies generally exhibit fewer adverse effects and longer half-lives compared to chimeric antibodies, contributing to better patient tolerance and therapeutic outcomes.
Immunogenicity and Patient Tolerance
Chimeric antibodies, composed of murine variable regions and human constant regions, exhibit higher immunogenicity compared to fully human monoclonal antibodies, leading to increased risk of anti-drug antibody (ADA) development and reduced patient tolerance. Monoclonal antibodies, especially fully human or humanized variants, minimize immune system recognition, resulting in improved patient tolerance and sustained therapeutic efficacy. Lower immunogenicity in monoclonal antibodies reduces infusion-related reactions and enhances the safety profile in long-term treatments.
Cost, Availability, and Manufacturing Challenges
Chimeric antibodies generally incur lower manufacturing costs due to simpler production processes compared to fully human monoclonal antibodies, which require advanced biotechnological methods and higher resource investment. Availability of chimeric antibodies is broader, as they have been established longer with numerous approved therapies, while monoclonal antibodies often face supply constraints linked to complex cell line development and stringent production standards. Manufacturing challenges for monoclonal antibodies include ensuring cell line stability and scalability, making them more expensive and less readily accessible than chimeric antibodies, which benefit from more robust and cost-efficient production systems.
Future Trends in Antibody Therapeutics
Future trends in antibody therapeutics emphasize the evolution from chimeric to fully human monoclonal antibodies, enhancing specificity and reducing immunogenicity. Advances in antibody engineering, including bispecific formats and antibody-drug conjugates, are accelerating the development of targeted therapies with improved efficacy. Integration of artificial intelligence in antibody design is expected to optimize therapeutic candidates, fostering personalized medicine approaches.
Chimeric Infographic
 libterm.com
libterm.com