Putrefaction is the natural decomposition process where organic matter breaks down due to microbial activity, resulting in the release of gases and unpleasant odors. This biological phenomenon significantly affects nutrient recycling in ecosystems and has critical implications in forensic science for determining time of death. Discover how putrefaction influences various scientific fields and what it means for your understanding of decomposition in the detailed article ahead.
Table of Comparison
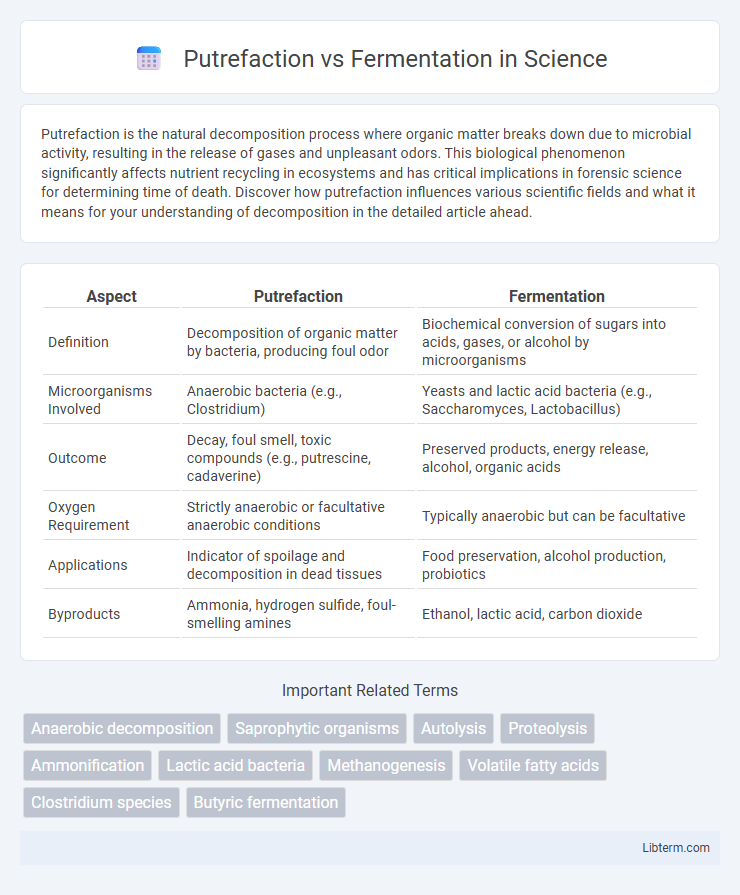
| Aspect | Putrefaction | Fermentation |
|---|---|---|
| Definition | Decomposition of organic matter by bacteria, producing foul odor | Biochemical conversion of sugars into acids, gases, or alcohol by microorganisms |
| Microorganisms Involved | Anaerobic bacteria (e.g., Clostridium) | Yeasts and lactic acid bacteria (e.g., Saccharomyces, Lactobacillus) |
| Outcome | Decay, foul smell, toxic compounds (e.g., putrescine, cadaverine) | Preserved products, energy release, alcohol, organic acids |
| Oxygen Requirement | Strictly anaerobic or facultative anaerobic conditions | Typically anaerobic but can be facultative |
| Applications | Indicator of spoilage and decomposition in dead tissues | Food preservation, alcohol production, probiotics |
| Byproducts | Ammonia, hydrogen sulfide, foul-smelling amines | Ethanol, lactic acid, carbon dioxide |
Understanding Putrefaction and Fermentation
Putrefaction is the microbial decomposition of organic matter, mainly proteins, resulting in the production of foul-smelling compounds such as ammonia, hydrogen sulfide, and methane. Fermentation is a metabolic process where microorganisms like yeast and bacteria convert sugars into alcohol or acids under anaerobic conditions, producing energy and various bio-products. Understanding these distinct biochemical pathways is essential for applications in food preservation, waste management, and biotechnology.
Key Differences Between Putrefaction and Fermentation
Putrefaction primarily involves the anaerobic decomposition of proteins by bacteria, resulting in foul-smelling compounds such as ammonia and hydrogen sulfide, whereas fermentation breaks down carbohydrates into simpler compounds like alcohol or lactic acid, often yielding beneficial products. Putrefaction occurs in dead organic matter leading to tissue decay and odor, while fermentation is a controlled enzymatic process used in food and beverage production. The microbial agents differ, with putrefaction dominated by proteolytic bacteria and fermentation driven by yeasts or lactic acid bacteria.
Biological Processes Involved
Putrefaction and fermentation are distinct biological processes driven by microbial activity breaking down organic matter under different conditions. Putrefaction involves anaerobic decomposition of proteins by bacteria, producing foul-smelling compounds like hydrogen sulfide and ammonia, commonly seen in decaying animal tissue. Fermentation primarily converts carbohydrates into alcohols, acids, or gases through enzymatic reactions by yeasts and bacteria, widely utilized in food production and energy metabolism.
Microorganisms Responsible for Each Process
Putrefaction primarily involves anaerobic bacteria such as Clostridium species and Bacteroides, which break down proteins in the absence of oxygen, producing foul-smelling compounds like hydrogen sulfide and ammonia. Fermentation is driven mainly by yeasts like Saccharomyces cerevisiae and lactic acid bacteria such as Lactobacillus, which convert sugars into alcohol or lactic acid under anaerobic conditions. These distinct microbial communities define the biochemical pathways and end products characteristic of putrefaction and fermentation processes.
Environmental Conditions Affecting Putrefaction and Fermentation
Putrefaction primarily occurs in anaerobic environments with higher temperatures, where bacteria decompose organic matter, producing foul odors and gases like hydrogen sulfide. Fermentation thrives in anaerobic conditions but favors moderate temperatures and specific microorganisms such as yeast or lactic acid bacteria, leading to the production of alcohol, acids, or gases without putrefactive odors. Moisture levels, pH, and oxygen availability significantly influence the rate and type of both putrefaction and fermentation processes in various ecosystems.
End Products and Byproducts
Putrefaction primarily produces foul-smelling compounds such as ammonia, hydrogen sulfide, and putrescine through the decomposition of proteins. Fermentation generates organic acids, alcohols, and gases like carbon dioxide as byproducts of carbohydrate breakdown. While putrefaction leads to toxic and malodorous substances, fermentation results in energy-rich compounds useful in food and biofuel industries.
Role in Food Preservation and Spoilage
Putrefaction causes food spoilage through the breakdown of proteins by anaerobic bacteria, producing foul odors and harmful substances, making food unsafe for consumption. Fermentation, driven by beneficial microbes like yeast and lactic acid bacteria, transforms sugars into alcohol or acids, enhancing flavor and extending shelf life in products like yogurt, sauerkraut, and wine. Understanding the microbial pathways differentiates putrefaction as a spoilage process while fermentation serves as a controlled preservation method.
Impacts on Human Health
Putrefaction releases toxic compounds such as ammonia, hydrogen sulfide, and cadaverine, which can cause severe food poisoning and pose significant health risks when ingested. Fermentation produces beneficial substances like lactic acid and probiotics that enhance gut health, improve digestion, and boost the immune system. Understanding these biochemical processes is crucial for food safety, as putrefaction indicates spoilage and harmful microbial activity, whereas fermentation often denotes controlled microbial activity with positive health effects.
Applications in Industry and Waste Management
Putrefaction, characterized by the anaerobic decomposition of proteins producing foul-smelling gases, is primarily applied in waste management for the degradation of organic refuse and sewage sludge. Fermentation, involving microbial conversion of carbohydrates into alcohol or acids, plays a crucial role in industries such as food production, biofuel manufacturing, and pharmaceuticals. Efficient harnessing of fermentation processes enables sustainable energy generation and bioproduct synthesis, while controlled use of putrefaction aids in organic waste reduction and nutrient recycling.
Summary Table: Putrefaction vs Fermentation
Putrefaction and fermentation are both anaerobic biochemical processes involving the breakdown of organic matter, but putrefaction primarily decomposes proteins into foul-smelling compounds like ammonia and hydrogen sulfide, while fermentation converts carbohydrates into alcohols or acids such as ethanol and lactic acid. Putrefaction typically occurs in dead animal tissue, producing gases and putrid odors, whereas fermentation is carried out by microorganisms like yeast and bacteria, useful in food production and preservation. Key differences include the substrates involved--proteins in putrefaction and carbohydrates in fermentation--and the resulting products, with putrefaction generating malodorous compounds and fermentation yielding energy-rich metabolites.
Putrefaction Infographic
 libterm.com
libterm.com