Isochoric processes occur at constant volume, meaning the system's volume does not change during the transformation. This concept is essential in thermodynamics, as it helps analyze how pressure and temperature evolve without expansion or compression. Explore the article to understand how isochoric conditions impact various physical and engineering applications.
Table of Comparison
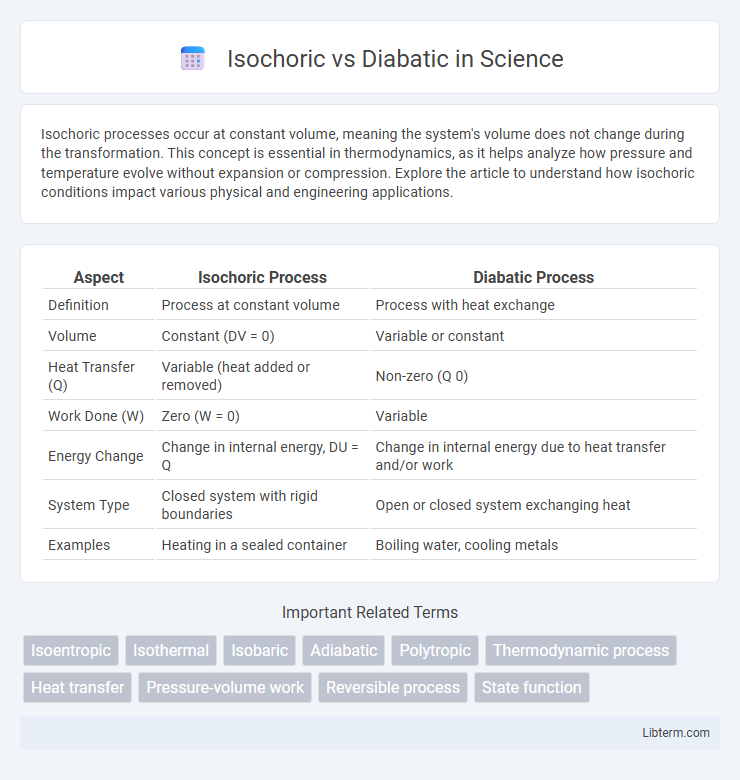
| Aspect | Isochoric Process | Diabatic Process |
|---|---|---|
| Definition | Process at constant volume | Process with heat exchange |
| Volume | Constant (DV = 0) | Variable or constant |
| Heat Transfer (Q) | Variable (heat added or removed) | Non-zero (Q 0) |
| Work Done (W) | Zero (W = 0) | Variable |
| Energy Change | Change in internal energy, DU = Q | Change in internal energy due to heat transfer and/or work |
| System Type | Closed system with rigid boundaries | Open or closed system exchanging heat |
| Examples | Heating in a sealed container | Boiling water, cooling metals |
Introduction to Isochoric and Diabatic Processes
Isochoric processes occur at constant volume, where no work is done on or by the system as the volume remains fixed, causing pressure and temperature to change accordingly. Diabatic processes involve heat transfer between the system and its surroundings, resulting in changes in internal energy and often affecting pressure, volume, and temperature simultaneously. Understanding these processes is essential in thermodynamics for analyzing energy exchange and system behavior under different constraints.
Fundamental Thermodynamic Concepts
Isochoric processes maintain constant volume, ensuring no work is done by the system as pressure changes, based on the thermodynamic principle \( W = P \Delta V \). Diabatic processes involve heat transfer across the system boundary, altering the internal energy and entropy according to the first and second laws of thermodynamics. These fundamental distinctions are critical in analyzing energy interactions in closed systems and designing thermodynamic cycles like Carnot and Otto engines.
Defining Isochoric Processes
Isochoric processes are thermodynamic transformations that occur at a constant volume, meaning the system's volume remains unchanged throughout the process. In these processes, no work is done by or on the system since work in thermodynamics is volume-dependent, but heat exchange may occur, altering the system's internal energy. Isochoric conditions are contrasted with diabatic processes, where heat exchange is involved, emphasizing that isochoric processes are a specific case of diabatic processes with fixed volume.
Understanding Diabatic Processes
Diabatic processes involve heat transfer between a system and its surroundings, causing a change in the system's internal energy and temperature. Unlike isochoric processes, where volume remains constant and no work is done, diabatic changes allow energy exchange through heat, impacting thermodynamic properties dynamically. Understanding diabatic behavior is critical for applications like climate modeling, engine thermodynamics, and energy systems analysis.
Mathematical Representation and Equations
Isochoric processes maintain constant volume, mathematically represented by \( \Delta V = 0 \) and the equation \( Q = \Delta U = n C_V \Delta T \), where \( Q \) is heat added, \( \Delta U \) is change in internal energy, \( n \) is number of moles, \( C_V \) is the molar heat capacity at constant volume, and \( \Delta T \) is temperature change. Diabatic processes involve heat exchange with the surroundings, quantified by the first law of thermodynamics \( \Delta U = Q - W \), where \( W \) is work done by or on the system, and \( Q \neq 0 \) accounts for heat transfer; the specific form of \( W \) depends on process conditions. The fundamental difference is that isochoric processes show zero work ( \( W = 0 \) ) due to constant volume, whereas diabatic processes allow \( W \neq 0 \), reflecting energy exchange through heat and work.
Key Differences: Isochoric vs Diabatic
Isochoric processes occur at constant volume, meaning no work is done by the system, while diabatic processes involve heat transfer between the system and its surroundings. In an isochoric process, pressure and temperature can change, but volume remains fixed, unlike diabatic processes where both heat flow and work interactions can affect the system's state. Understanding these distinctions is crucial for thermodynamic analysis and engineering applications, as they dictate different energy exchange mechanisms and system behavior.
Practical Applications in Engineering
Isochoric processes, characterized by constant volume, are crucial in applications such as internal combustion engines and refrigeration cycles, where maintaining a fixed volume facilitates precise control of pressure and temperature changes. Diabatic processes, involving heat transfer, are fundamental in heat exchangers, HVAC systems, and thermal power plants, where managing heat flow optimizes energy efficiency and system performance. Engineering designs leverage isochoric conditions to ensure structural integrity under pressure, while diabatic principles enable effective thermal management across various industrial systems.
Advantages and Limitations of Each Process
Isochoric processes maintain constant volume, enabling precise control of internal energy changes without work done on or by the system, which is advantageous in calibration settings but limits practical energy transfer applications due to the fixed volume constraint. Diabatic processes involve heat exchange with the surroundings, allowing efficient thermal energy transfer and temperature regulation in various systems, yet they often require complex insulation and control mechanisms to manage undesired heat losses. The choice between isochoric and diabatic processes hinges on the specific requirements for energy control versus heat interaction, balancing operational simplicity against thermal management needs.
Real-World Examples and Case Studies
Isochoric processes, characterized by constant volume heat exchange, are exemplified in rigid container heating such as pressure cookers where volume remains fixed while temperature and pressure rise. Diabatic processes involving heat transfer occur in real-world heat exchangers, like radiators and condensers, where fluid properties change due to thermal energy exchange without mechanical work. Case studies in automotive engines often analyze isochoric combustion phases, while HVAC system designs incorporate diabatic heat transfer principles for efficient temperature control.
Summary and Comparative Analysis
Isochoric processes occur at constant volume, preventing work output due to zero displacement, while diabatic processes involve heat transfer to or from the system, altering internal energy without constraints on volume or pressure. Isochoric processes are typically analyzed in closed systems with rigid boundaries, whereas diabatic processes apply broadly, including open and closed systems experiencing heat exchange. Comparative analysis reveals isochoric as a specific subset where volume remains fixed, contrasting with diabatic conditions that prioritize thermal interactions, making them central in thermodynamic cycles and energy transfer studies.
Isochoric Infographic
 libterm.com
libterm.com