Strong electrolytes completely dissociate into ions when dissolved in water, enabling efficient electrical conductivity. Common examples include salts like sodium chloride, strong acids such as hydrochloric acid, and strong bases like sodium hydroxide. Explore the rest of this article to understand how strong electrolytes influence chemical reactions and practical applications.
Table of Comparison
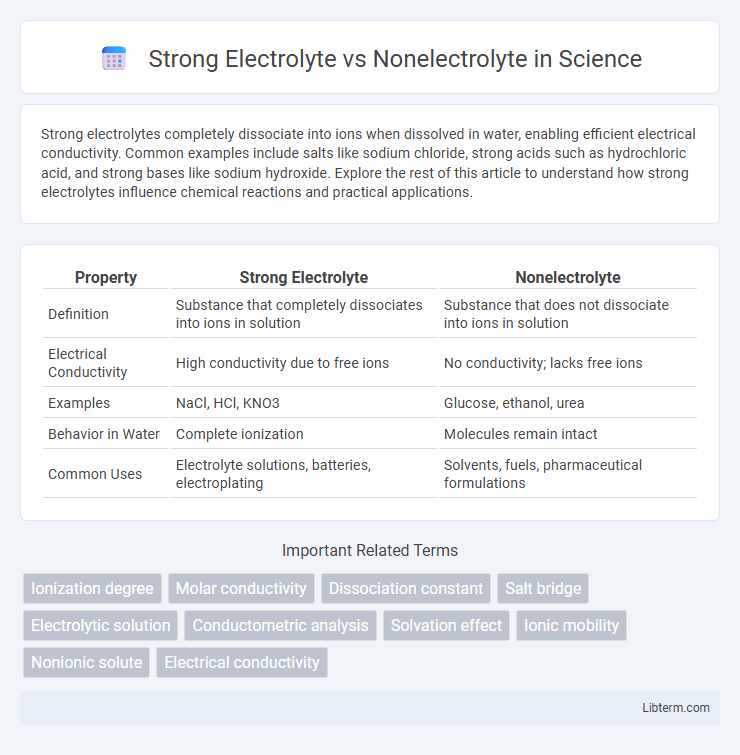
| Property | Strong Electrolyte | Nonelectrolyte |
|---|---|---|
| Definition | Substance that completely dissociates into ions in solution | Substance that does not dissociate into ions in solution |
| Electrical Conductivity | High conductivity due to free ions | No conductivity; lacks free ions |
| Examples | NaCl, HCl, KNO3 | Glucose, ethanol, urea |
| Behavior in Water | Complete ionization | Molecules remain intact |
| Common Uses | Electrolyte solutions, batteries, electroplating | Solvents, fuels, pharmaceutical formulations |
Introduction to Electrolytes
Strong electrolytes completely dissociate into ions when dissolved in water, enabling efficient electrical conductivity, whereas nonelectrolytes do not ionize and thus do not conduct electricity. Common strong electrolytes include salts like sodium chloride (NaCl) and strong acids such as hydrochloric acid (HCl), which produce a high concentration of free ions in solution. Understanding the distinction between strong electrolytes and nonelectrolytes is crucial for applications in chemistry, biology, and industry where ion concentration influences reactions and conductivity.
Defining Strong Electrolytes
Strong electrolytes are substances that completely dissociate into ions when dissolved in water, resulting in a highly conductive solution. Common examples include sodium chloride (NaCl), hydrochloric acid (HCl), and potassium hydroxide (KOH), which produce a large concentration of free ions. Unlike nonelectrolytes, such as glucose or ethanol, strong electrolytes enable efficient electrical conductivity due to their full ionization in aqueous solutions.
Understanding Nonelectrolytes
Nonelectrolytes are substances that do not dissociate into ions when dissolved in water, resulting in no electrical conductivity. Unlike strong electrolytes, which completely ionize and conduct electricity efficiently, nonelectrolytes remain as neutral molecules in solution. Common examples of nonelectrolytes include sugar and ethanol, which dissolve without producing charged particles.
Key Differences Between Strong Electrolytes and Nonelectrolytes
Strong electrolytes completely dissociate into ions in aqueous solutions, resulting in high electrical conductivity, whereas nonelectrolytes do not ionize and thus conduct virtually no electricity. Strong electrolytes include substances like sodium chloride (NaCl) and hydrochloric acid (HCl), while nonelectrolytes consist of compounds such as glucose and ethanol that dissolve as intact molecules. The degree of ionization directly influences their behavior in solution, impacting properties like osmotic pressure and colligative effects.
Examples of Strong Electrolytes
Strong electrolytes, such as sodium chloride (NaCl), potassium nitrate (KNO3), and hydrochloric acid (HCl), completely dissociate into ions in aqueous solutions, resulting in high electrical conductivity. These substances include most soluble salts, strong acids like sulfuric acid (H2SO4), and strong bases such as sodium hydroxide (NaOH). Their ability to produce a large concentration of ions distinguishes them from nonelectrolytes, which do not ionize and thus fail to conduct electricity effectively.
Examples of Nonelectrolytes
Nonelectrolytes like glucose, urea, and ethanol do not dissociate into ions in aqueous solutions, resulting in no electrical conductivity. Unlike strong electrolytes such as sodium chloride or hydrochloric acid, which completely ionize and conduct electricity efficiently, nonelectrolytes remain intact molecules. These substances are commonly used in biological and chemical processes where non-conductive solutions are required.
Electrical Conductivity Comparison
Strong electrolytes, such as sodium chloride and potassium hydroxide, fully dissociate into ions in aqueous solutions, resulting in high electrical conductivity. Nonelectrolytes like sugar or ethanol do not ionize in solution, leading to negligible or no electrical conductivity. The direct correlation between ion concentration and electrical conductivity highlights the significant difference in conductance between strong electrolytes and nonelectrolytes.
Real-World Applications
Strong electrolytes, such as sodium chloride and hydrochloric acid, fully dissociate into ions in aqueous solutions, making them essential for applications requiring efficient electrical conductivity, including batteries, electroplating, and medical IV fluids. Nonelectrolytes like glucose and ethanol do not ionize in water, making them ideal for applications where conductivity is undesirable, such as in pharmaceuticals and food products. Understanding the difference between strong electrolytes and nonelectrolytes allows engineers and scientists to select appropriate substances for chemical sensors, water treatment processes, and industrial manufacturing.
Experimental Identification Methods
Strong electrolytes fully dissociate into ions in aqueous solution, resulting in high electrical conductivity, which can be experimentally identified using conductometric measurements. Nonelectrolytes do not produce ions when dissolved and therefore exhibit negligible conductivity, distinguishable through low or near-zero conductance values. Electrochemical methods, such as measuring solution resistance or using conductivity meters, are key techniques in differentiating strong electrolytes from nonelectrolytes.
Summary and Key Takeaways
Strong electrolytes completely dissociate into ions in aqueous solutions, resulting in high electrical conductivity, while nonelectrolytes do not dissociate and thus do not conduct electricity. Key takeaways include the fact that strong electrolytes, such as sodium chloride and hydrochloric acid, produce abundant free ions, making them essential in processes requiring efficient ion transport, whereas nonelectrolytes like glucose and urea remain intact molecules and have negligible effect on conductivity. Understanding the distinction is crucial in fields like chemistry and biology for predicting solution behavior and designing industrial applications.
Strong Electrolyte Infographic
 libterm.com
libterm.com