Isothermal processes maintain a constant temperature throughout a system, ensuring energy exchange occurs solely as work rather than heat change. This principle is fundamental in thermodynamics, especially in understanding gas behavior during compression or expansion. Explore the rest of the article to learn how isothermal conditions impact various scientific and engineering applications.
Table of Comparison
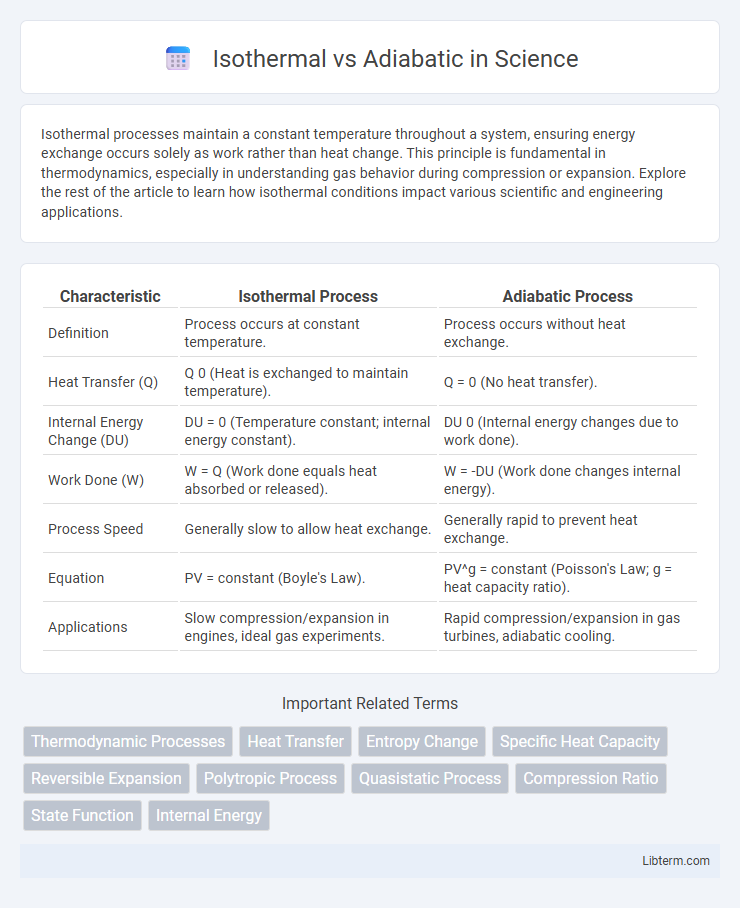
| Characteristic | Isothermal Process | Adiabatic Process |
|---|---|---|
| Definition | Process occurs at constant temperature. | Process occurs without heat exchange. |
| Heat Transfer (Q) | Q 0 (Heat is exchanged to maintain temperature). | Q = 0 (No heat transfer). |
| Internal Energy Change (DU) | DU = 0 (Temperature constant; internal energy constant). | DU 0 (Internal energy changes due to work done). |
| Work Done (W) | W = Q (Work done equals heat absorbed or released). | W = -DU (Work done changes internal energy). |
| Process Speed | Generally slow to allow heat exchange. | Generally rapid to prevent heat exchange. |
| Equation | PV = constant (Boyle's Law). | PV^g = constant (Poisson's Law; g = heat capacity ratio). |
| Applications | Slow compression/expansion in engines, ideal gas experiments. | Rapid compression/expansion in gas turbines, adiabatic cooling. |
Introduction to Isothermal and Adiabatic Processes
Isothermal and adiabatic processes are fundamental concepts in thermodynamics describing how systems exchange heat and work. Isothermal processes occur at a constant temperature, allowing heat transfer to maintain thermal equilibrium, while adiabatic processes happen without heat exchange, resulting in changes in internal energy due to work done on or by the system. Understanding these processes is essential for analyzing thermodynamic cycles, engine efficiency, and gas behavior under different thermodynamic constraints.
Defining Isothermal Processes
Isothermal processes maintain a constant temperature throughout the thermodynamic transformation, ensuring heat exchange with the surroundings balances any work done by the system. In these processes, the internal energy of an ideal gas remains unchanged according to the first law of thermodynamics, as energy transfer occurs solely as heat. Contrastingly, adiabatic processes involve no heat exchange, causing temperature to vary with pressure and volume changes in the system.
Understanding Adiabatic Processes
Adiabatic processes involve the transfer of energy as work without heat exchange with the surroundings, maintaining constant entropy within the system. These processes are characterized by rapid compression or expansion in gases, where temperature changes result solely from pressure variations. Understanding adiabatic dynamics is crucial in thermodynamics for modeling engines, atmospheric phenomena, and refrigeration cycles.
Key Differences Between Isothermal and Adiabatic
Isothermal processes maintain a constant temperature by allowing heat exchange with the surroundings, whereas adiabatic processes prevent heat transfer, causing temperature to change during expansion or compression. In isothermal conditions, internal energy remains constant, while in adiabatic processes, internal energy varies with work done on or by the system. These differences significantly impact thermodynamic analyses, especially in applications like engines and compressors where heat transfer and temperature control are critical.
Thermodynamic Principles Involved
Isothermal processes maintain constant temperature by allowing heat exchange with the surroundings, ensuring internal energy remains unchanged according to the first law of thermodynamics. Adiabatic processes occur without heat transfer, resulting in temperature changes within the system due to work done on or by the gas, governed by the adiabatic condition \( PV^\gamma = \text{constant} \). Understanding these distinct thermodynamic principles is essential for analyzing energy transformations in engines, compressors, and refrigerators.
Real-World Examples of Isothermal Processes
Isothermal processes occur when a system's temperature remains constant despite energy exchange, commonly seen in the slow compression or expansion of gases in piston engines and refrigeration cycles. Industrial applications include chemical reactors maintaining steady temperature during gas absorption and biological systems like human lungs where gas exchange occurs isothermally. These real-world examples highlight the practical use of isothermal transformations in maintaining thermal equilibrium while facilitating energy transfer.
Real-World Examples of Adiabatic Processes
Adiabatic processes occur when a system undergoes a change without heat exchange with its surroundings, commonly observed in natural phenomena such as the rapid expansion of air in thunderstorm clouds, leading to adiabatic cooling and cloud formation. In industrial applications, adiabatic compression is utilized in gas turbines and refrigeration cycles, where the temperature of the gas increases due to pressure changes without heat transfer. The Earth's atmosphere consistently demonstrates adiabatic lapse rates, where rising air parcels cool at a specific rate dictated by pressure changes, essential for weather prediction models and understanding convection currents.
Advantages and Limitations of Each Process
Isothermal processes maintain constant temperature, allowing for maximum work extraction in systems like heat engines, but require slow compression or expansion to avoid temperature changes, limiting speed and practicality. Adiabatic processes involve no heat exchange, enabling rapid changes and energy efficiency in insulated systems, yet they often result in significant temperature variations that can impact material properties and system stability. The choice between isothermal and adiabatic processes depends on specific application needs, balancing efficiency, speed, and thermal management.
Applications in Engineering and Industry
Isothermal processes, maintaining constant temperature, are crucial in chemical reactors and refrigeration systems where thermal equilibrium ensures steady reactions and energy efficiency. Adiabatic processes, involving no heat exchange, are pivotal in gas turbines and compressors for rapid expansion or compression, optimizing work output and efficiency. Engineering applications leverage isothermal conditions for controlled chemical synthesis and adiabatic conditions for high-speed thermodynamic cycles in power generation and propulsion.
Summary: Choosing Between Isothermal and Adiabatic
Choosing between isothermal and adiabatic processes depends on heat exchange and system conditions. Isothermal processes maintain a constant temperature by allowing heat transfer, ideal for slow operations where temperature control is critical. Adiabatic processes prevent heat exchange, resulting in temperature changes, and are suited for rapid processes or insulated systems where energy conservation is essential.
Isothermal Infographic
 libterm.com
libterm.com