Constitutional isomers are molecules that share the same molecular formula but differ in the connectivity of their atoms, resulting in distinct structural arrangements and properties. These variations affect physical and chemical behaviors, making them important in fields like organic chemistry and pharmaceuticals. Discover more about how constitutional isomers impact molecular function and applications in the rest of this article.
Table of Comparison
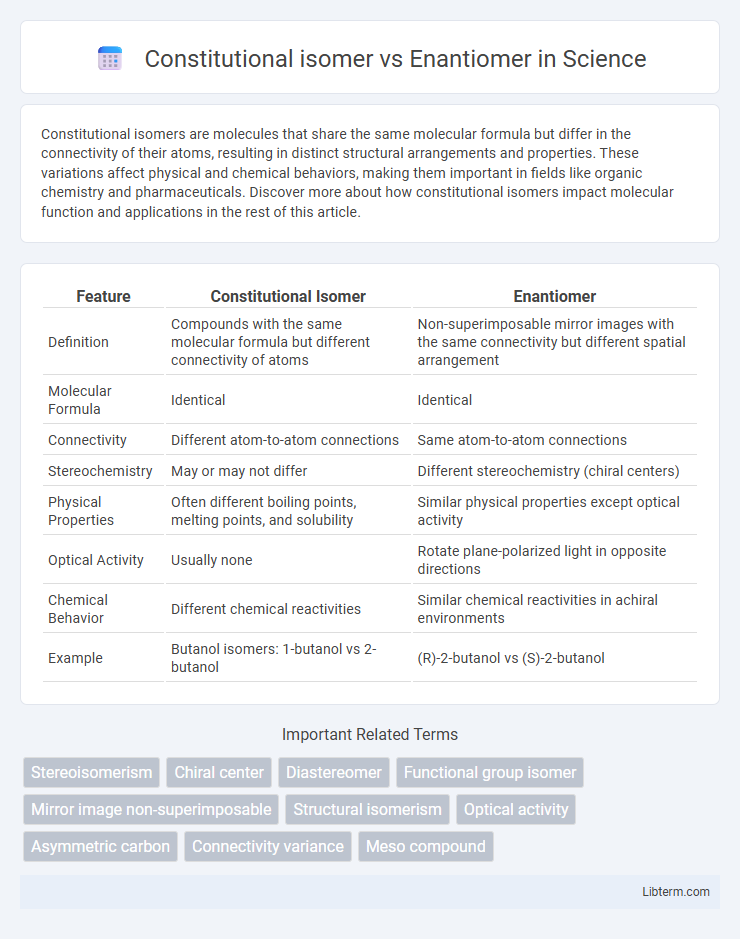
| Feature | Constitutional Isomer | Enantiomer |
|---|---|---|
| Definition | Compounds with the same molecular formula but different connectivity of atoms | Non-superimposable mirror images with the same connectivity but different spatial arrangement |
| Molecular Formula | Identical | Identical |
| Connectivity | Different atom-to-atom connections | Same atom-to-atom connections |
| Stereochemistry | May or may not differ | Different stereochemistry (chiral centers) |
| Physical Properties | Often different boiling points, melting points, and solubility | Similar physical properties except optical activity |
| Optical Activity | Usually none | Rotate plane-polarized light in opposite directions |
| Chemical Behavior | Different chemical reactivities | Similar chemical reactivities in achiral environments |
| Example | Butanol isomers: 1-butanol vs 2-butanol | (R)-2-butanol vs (S)-2-butanol |
Introduction to Isomerism
Isomerism occurs when molecules share the same molecular formula but differ in structural arrangement or spatial orientation. Constitutional isomers, also known as structural isomers, vary in the connectivity of atoms, resulting in distinct molecular frameworks. Enantiomers are stereoisomers that are non-superimposable mirror images, differing in the spatial configuration of chiral centers while maintaining the same atomic connectivity.
Definition of Constitutional Isomers
Constitutional isomers, also known as structural isomers, are compounds with the same molecular formula but different connectivity of their atoms, resulting in distinct chemical structures and properties. These isomers differ in the sequence in which atoms are bonded, leading to variations such as chain isomerism, position isomerism, and functional group isomerism. Unlike enantiomers, constitutional isomers do not exhibit mirror-image stereochemistry but rather differ in the arrangement of their atomic framework.
Definition of Enantiomers
Enantiomers are stereoisomers that are non-superimposable mirror images of each other due to the presence of a chiral center, typically a carbon atom bonded to four different substituents. Unlike constitutional isomers, which differ in the connectivity of atoms, enantiomers have identical molecular formulas and connectivity but differ in the spatial arrangement of atoms, leading to distinct optical activities. Enantiomers exhibit chirality and rotate plane-polarized light in opposite directions, playing a crucial role in pharmacology and biochemical interactions.
Structural Differences: Constitutional Isomer vs Enantiomer
Constitutional isomers differ in the connectivity of their atoms, resulting in different bonding sequences and distinct molecular structures. Enantiomers are stereoisomers with the same atomic connectivity but differ in the spatial arrangement of atoms, specifically as non-superimposable mirror images. Structural differences in constitutional isomers affect molecular formula and properties, while enantiomers share the same formula but have unique chiral configurations affecting optical activity.
Physical and Chemical Properties Comparison
Constitutional isomers differ in the connectivity of atoms, resulting in distinct physical properties such as boiling points, melting points, and densities due to variations in molecular structure and intermolecular forces. Enantiomers are stereoisomers that are non-superimposable mirror images, exhibiting identical physical properties except for their interaction with plane-polarized light and chiral environments, leading to differences in optical rotation and biological activity. Chemically, constitutional isomers often have different reactivities due to functional group placement, whereas enantiomers typically show identical reactivity unless they interact with other chiral compounds or catalysts.
Stereochemistry and Optical Activity
Constitutional isomers differ in the connectivity of atoms, resulting in distinct structural arrangements without necessarily impacting optical activity, whereas enantiomers are stereoisomers that are non-superimposable mirror images, exhibiting opposite optical activity due to their chiral centers. Stereochemistry plays a crucial role in enantiomers by determining the spatial arrangement around asymmetric carbon atoms, directly influencing their ability to rotate plane-polarized light either clockwise or counterclockwise. Optical activity is a defining feature of enantiomers, measured as specific rotation, while constitutional isomers typically lack optical activity unless they contain chiral centers themselves.
Methods for Identifying Isomers
Constitutional isomers are identified primarily through techniques such as nuclear magnetic resonance (NMR) spectroscopy and infrared (IR) spectroscopy, which reveal differences in connectivity and functional groups. Enantiomers require chiral-specific methods like polarimetry, chiral chromatography, and circular dichroism (CD) spectroscopy to distinguish between mirror-image structures. Mass spectrometry alone cannot differentiate these isomers, emphasizing the need for stereochemical analysis in enantiomer identification.
Real-Life Examples and Applications
Constitutional isomers differ in the connectivity of atoms, exemplified by glucose and fructose, which have distinct metabolic pathways in the human body. Enantiomers are mirror-image molecules like the drug thalidomide, where one enantiomer treats morning sickness while the other causes severe birth defects. In pharmaceuticals, distinguishing between constitutional isomers and enantiomers affects drug efficacy and safety, making stereochemistry crucial in drug design and regulatory approval.
Importance in Pharmaceutical Industry
Constitutional isomers differ in the connectivity of atoms, leading to distinct chemical and physical properties that can impact drug efficacy and safety in pharmaceutical formulations. Enantiomers are mirror-image isomers with identical connectivity but different spatial arrangements, often exhibiting vastly different biological activities and metabolic pathways critical for drug design and therapeutic outcomes. Understanding the distinction and behavior of these isomers is essential for optimizing drug development, regulatory approval, and minimizing adverse effects in the pharmaceutical industry.
Conclusion: Key Differences and Significance
Constitutional isomers differ in the connectivity of atoms, resulting in distinct structural formulas and unique physical and chemical properties, while enantiomers are chiral molecules that are non-superimposable mirror images with identical connectivity but different spatial arrangements. The significance of constitutional isomers lies in their diversity of compounds and functional groups, influencing reactivity and synthesis pathways. Enantiomers play a crucial role in pharmaceuticals and biochemistry due to their distinct interactions with polarized light and biological receptors, impacting drug efficacy and safety.
Constitutional isomer Infographic
 libterm.com
libterm.com