Direct titration involves adding a titrant directly to the analyte until the reaction reaches the equivalence point, enabling precise determination of concentration. This method is widely used in analytical chemistry due to its simplicity and accuracy for various substances. Discover how direct titration can simplify your laboratory analyses by reading the full article.
Table of Comparison
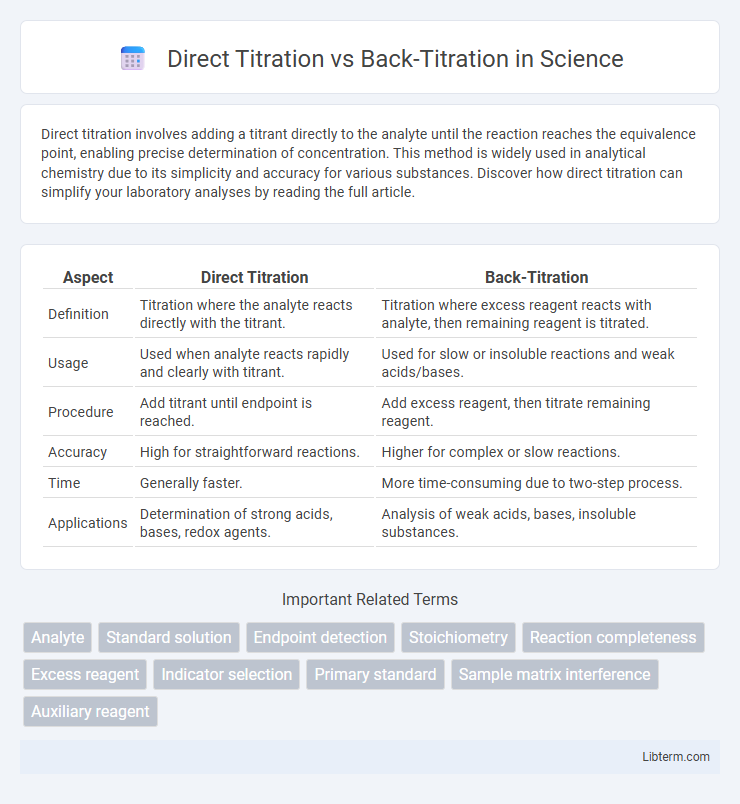
| Aspect | Direct Titration | Back-Titration |
|---|---|---|
| Definition | Titration where the analyte reacts directly with the titrant. | Titration where excess reagent reacts with analyte, then remaining reagent is titrated. |
| Usage | Used when analyte reacts rapidly and clearly with titrant. | Used for slow or insoluble reactions and weak acids/bases. |
| Procedure | Add titrant until endpoint is reached. | Add excess reagent, then titrate remaining reagent. |
| Accuracy | High for straightforward reactions. | Higher for complex or slow reactions. |
| Time | Generally faster. | More time-consuming due to two-step process. |
| Applications | Determination of strong acids, bases, redox agents. | Analysis of weak acids, bases, insoluble substances. |
Introduction to Titration Methods
Direct titration involves the gradual addition of a titrant to a known volume of analyte until the reaction reaches an endpoint, allowing for straightforward concentration determination. Back-titration is used when the analyte is insoluble or reacts slowly, by adding an excess reagent and then titrating the remaining excess with a second titrant, offering enhanced accuracy for complex samples. Both methods rely on precise endpoint detection and are essential techniques in volumetric chemical analysis.
What is Direct Titration?
Direct titration is an analytical technique where a titrant of known concentration is added directly to a solution containing the analyte until the reaction reaches the equivalence point, indicated by a color change or instrumental signal. This method is commonly used for strong acid-strong base reactions, enabling straightforward calculation of the analyte concentration from the volume of titrant consumed. It is preferred for its simplicity and accuracy when the analyte reacts quickly and completely with the titrant.
What is Back-Titration?
Back-titration is an analytical technique used when the analyte is difficult to titrate directly due to slow reaction rates, insolubility, or lack of suitable indicators. It involves adding an excess of standard reagent to the analyte, then titrating the unreacted reagent with a second standard solution to determine the analyte concentration indirectly. This method enhances accuracy and is commonly applied in complex acid-base, redox, and precipitation reactions.
Key Differences: Direct vs Back-Titration
Direct titration involves the gradual addition of a titrant to a known volume of analyte until the reaction reaches an endpoint, typically detected by a color change or pH indicator. Back-titration, on the other hand, begins by adding an excess of standard reagent to the analyte and then titrating the remaining reagent with a second titrant to calculate the analyte concentration indirectly. Direct titration is faster and simpler for clear endpoint reactions, while back-titration is preferred for analytes that react slowly or have ambiguous endpoints.
Advantages of Direct Titration
Direct titration offers greater simplicity and speed by allowing the endpoint to be determined in a single step, reducing analysis time. It provides higher accuracy and precision since the titrant directly reacts with the analyte, minimizing errors from additional reagents. This method is ideal for samples with clear, rapid reactions, facilitating straightforward endpoint detection through indicators or potentiometric means.
Benefits of Back-Titration
Back-titration allows accurate analysis of substances that react slowly or are insoluble, ensuring precise endpoint determination. It is particularly beneficial when the direct titration endpoint is difficult to observe or when the analyte is present in low concentrations. This method enhances flexibility and accuracy in complex chemical analysis and food testing scenarios.
Choosing the Right Titration Technique
Choosing between direct titration and back-titration depends on the nature of the analyte and the required precision. Direct titration is ideal for clear, rapid reactions where the endpoint is easily identified, making it suitable for strong acid-base reactions and transparent solutions. Back-titration is preferred for reactions with slow kinetics, insoluble products, or when the analyte is volatile, ensuring accurate quantification by titrating the excess reagent.
Common Applications in Chemistry
Direct titration is commonly used for analyzing strong acids or bases where the endpoint is easily detected, such as determining the concentration of hydrochloric acid using a strong base like sodium hydroxide. Back-titration is applied when the analyte is insoluble, volatile, or reacts slowly, for instance, in calcium carbonate content determination in cement or antacid tablets by adding excess acid and titrating the remaining acid with a base. Both methods are essential in pharmaceutical, environmental, and food chemistry for accurate quantification of substances with varying chemical properties.
Limitations and Challenges
Direct titration often faces limitations in analyzing substances with slow or incomplete reactions, weak or obscure endpoints, and samples with complex matrices that interfere with indicator visibility. Back-titration addresses some of these challenges by enabling the quantification of analytes that react poorly or have ambiguous endpoints; however, it introduces complexities such as increased procedural steps, potential errors from excess reagent measurement, and the need for precise secondary titrations. Both methods require careful selection based on sample type, reaction kinetics, and the availability of suitable titrants to minimize inaccuracies and ensure reliable analytical results.
Summary: Which Titration Method to Use?
Direct titration is ideal when the analyte reacts quickly and clearly with the titrant, providing straightforward endpoint detection, making it suitable for simple acid-base or redox reactions. Back-titration is preferred when the analyte is insoluble, slow to react, or when the endpoint is difficult to observe, as it involves adding an excess reagent and titrating the remaining amount. Choosing the appropriate method depends on the reaction characteristics, solubility, and the ease of detecting the equivalence point to ensure accurate and efficient analysis.
Direct Titration Infographic
 libterm.com
libterm.com