Crystalline materials exhibit a highly ordered atomic structure that defines their unique physical properties, such as hardness, transparency, and thermal conductivity. Understanding the molecular arrangement within crystalline solids is essential for advancements in electronics, optics, and pharmaceuticals. Explore the full article to discover how crystalline structures impact your everyday technology and innovations.
Table of Comparison
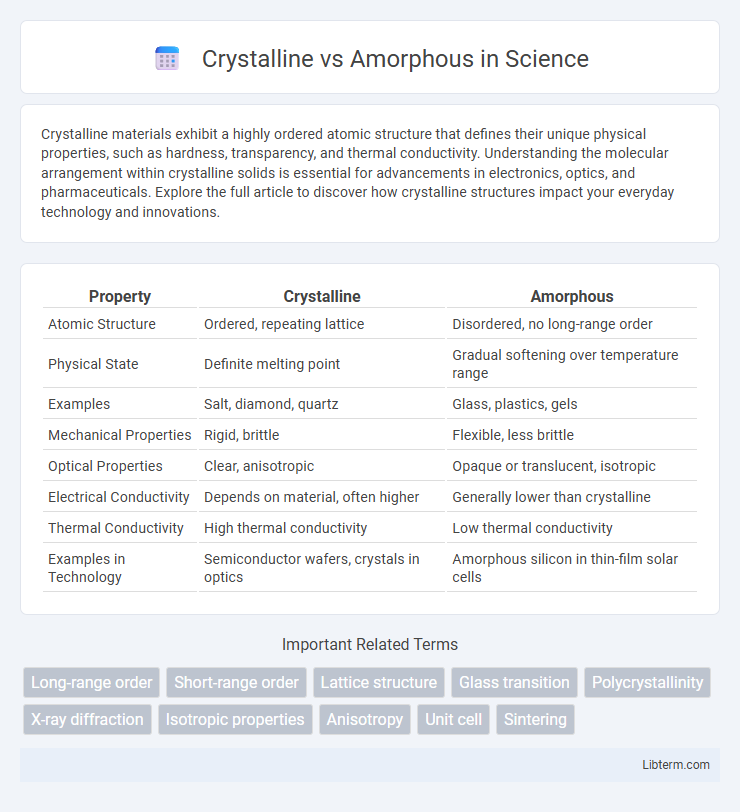
| Property | Crystalline | Amorphous |
|---|---|---|
| Atomic Structure | Ordered, repeating lattice | Disordered, no long-range order |
| Physical State | Definite melting point | Gradual softening over temperature range |
| Examples | Salt, diamond, quartz | Glass, plastics, gels |
| Mechanical Properties | Rigid, brittle | Flexible, less brittle |
| Optical Properties | Clear, anisotropic | Opaque or translucent, isotropic |
| Electrical Conductivity | Depends on material, often higher | Generally lower than crystalline |
| Thermal Conductivity | High thermal conductivity | Low thermal conductivity |
| Examples in Technology | Semiconductor wafers, crystals in optics | Amorphous silicon in thin-film solar cells |
Introduction to Crystalline and Amorphous Materials
Crystalline materials possess a highly ordered atomic structure with repeating patterns extending in all three spatial dimensions, resulting in distinct melting points and sharp diffraction patterns. Amorphous materials lack this long-range order, exhibiting a random atomic arrangement that leads to gradual softening over a temperature range and broad diffraction patterns. Understanding the structural differences between crystalline and amorphous materials is crucial for tailoring their mechanical, optical, and thermal properties in applications such as semiconductors, glasses, and polymers.
Defining Crystalline Structures
Crystalline structures exhibit a highly ordered arrangement of atoms or molecules, forming a repeating pattern throughout the material, which is characterized by defined geometric shapes and long-range order. This precise atomic alignment results in distinct physical properties such as sharp melting points, anisotropy, and well-defined cleavage planes. In contrast, amorphous materials lack this regular pattern, leading to isotropic behavior and gradual softening without a sharp melting point.
Understanding Amorphous Structures
Amorphous structures lack the long-range order characteristic of crystalline materials, resulting in atoms arranged randomly rather than in a repeating lattice. This disorder leads to unique physical properties such as isotropy and variable density, influencing applications in glasses, polymers, and thin-film technologies. Understanding atomic-scale irregularities in amorphous solids is critical for optimizing mechanical strength, optical clarity, and electrical conductivity in advanced material design.
Key Differences Between Crystalline and Amorphous Solids
Crystalline solids exhibit a well-ordered, repeating atomic structure resulting in sharp melting points and high rigidity, whereas amorphous solids lack a long-range order, leading to gradual softening over a range of temperatures and increased flexibility. The uniform lattice arrangement in crystalline materials provides distinct cleavage planes, contrasting with the random atomic distribution in amorphous solids that results in isotropic properties. Thermal conductivity and mechanical strength are generally higher in crystalline solids due to their structured atomic alignment, unlike the often lower conductivity and variable strength seen in amorphous counterparts.
Physical Properties Comparison
Crystalline materials exhibit a well-ordered atomic structure resulting in defined melting points, high density, and anisotropic mechanical properties, whereas amorphous materials lack long-range order, leading to broad melting ranges, lower density, and isotropic behavior. Crystalline solids typically have higher thermal and electrical conductivity due to their regular lattice arrangement, while amorphous solids show poorer conductivity owing to irregular atomic spacing. The rigidity and hardness of crystalline substances contrast with the generally more flexible and less brittle nature of amorphous solids, which impacts their applications in electronics and materials science.
Thermal and Electrical Conductivity Analysis
Crystalline materials exhibit high thermal and electrical conductivity due to their well-ordered atomic lattice structure, which facilitates efficient electron and phonon transport. Amorphous materials, lacking long-range order, display significantly lower conductivity because atomic irregularities scatter charge carriers and phonons, impeding transport. The difference in conductivity between crystalline silicon (~150 W/m*K thermal conductivity) and amorphous silicon (~1.5 W/m*K) underscores the impact of structural order on these properties.
Applications of Crystalline Materials
Crystalline materials, characterized by their ordered atomic structure, are essential in electronics, optics, and aerospace due to their predictable mechanical and thermal properties. Their use in semiconductors, such as silicon crystals, enables efficient electron flow critical for microchips and solar cells. The high strength and thermal resistance of crystalline metals also make them ideal for structural components in aerospace engineering.
Applications of Amorphous Materials
Amorphous materials, unlike their crystalline counterparts, exhibit a disordered atomic structure that provides unique properties such as high strength, flexibility, and isotropic behavior, making them ideal for applications in aerospace, electronics, and biomedical devices. Their ability to efficiently absorb electromagnetic waves enables use in transformers, inductors, and magnetic shielding, enhancing energy efficiency and reducing noise. The lack of grain boundaries improves corrosion resistance and wear properties, promoting their use in protective coatings and advanced optical devices.
Advantages and Disadvantages of Each Structure
Crystalline structures offer high stability, predictable melting points, and superior mechanical strength due to their ordered atomic arrangement, making them ideal for applications requiring durability. Amorphous structures, lacking long-range order, provide advantages such as increased optical transparency and flexibility, but they often suffer from lower thermal stability and mechanical strength. The choice between crystalline and amorphous materials depends on the specific requirements of thermal performance, mechanical properties, and optical clarity for targeted industrial or technological applications.
Conclusion: Choosing Between Crystalline and Amorphous
Choosing between crystalline and amorphous materials depends on the desired properties and application requirements. Crystalline substances offer high structural order, resulting in predictable mechanical strength and efficient charge transport, ideal for electronics and structural components. Amorphous materials, with their disordered atomic arrangement, provide flexibility, easier manufacturing, and unique optical properties, making them suitable for thin-film coatings and flexible devices.
Crystalline Infographic
 libterm.com
libterm.com