Statistical methods provide powerful tools for analyzing data trends, making predictions, and supporting informed decision-making in various fields. Mastering these techniques enhances your ability to interpret complex information and derive meaningful insights. Explore the rest of the article to discover practical applications and tips for leveraging statistics effectively.
Table of Comparison
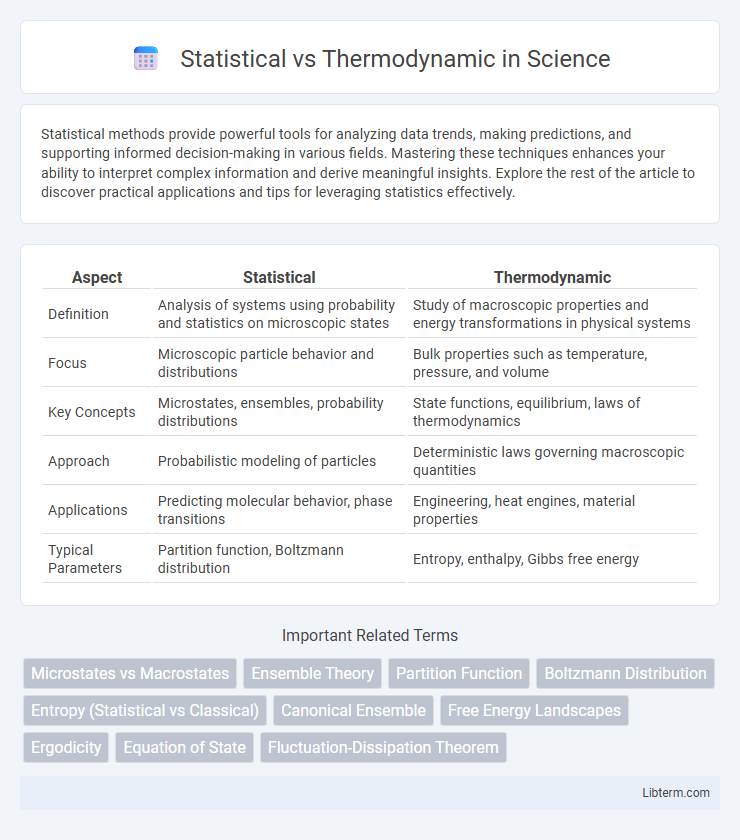
| Aspect | Statistical | Thermodynamic |
|---|---|---|
| Definition | Analysis of systems using probability and statistics on microscopic states | Study of macroscopic properties and energy transformations in physical systems |
| Focus | Microscopic particle behavior and distributions | Bulk properties such as temperature, pressure, and volume |
| Key Concepts | Microstates, ensembles, probability distributions | State functions, equilibrium, laws of thermodynamics |
| Approach | Probabilistic modeling of particles | Deterministic laws governing macroscopic quantities |
| Applications | Predicting molecular behavior, phase transitions | Engineering, heat engines, material properties |
| Typical Parameters | Partition function, Boltzmann distribution | Entropy, enthalpy, Gibbs free energy |
Introduction to Statistical and Thermodynamic Approaches
Statistical approaches analyze physical systems by evaluating the collective behavior of individual particles using probabilities and microstates, providing detailed insights into microscopic interactions. Thermodynamic methods focus on macroscopic properties such as temperature, pressure, and volume, using empirical laws to describe system behavior without detailing particle-level dynamics. Both approaches complement each other, with statistical mechanics bridging microscopic particle distributions and macroscopic thermodynamic observables.
Defining Statistical Methods in Science
Statistical methods in science involve the collection, analysis, and interpretation of data to uncover patterns and infer conclusions about populations from samples, emphasizing probability distributions and inferential techniques. These methods rely on statistical models and hypothesis testing to quantify uncertainty, enabling scientists to make predictions and validate experimental outcomes. Unlike thermodynamic approaches, which focus on energy transformations and physical laws governing systems, statistical methods provide a probabilistic framework critical for experimental design and data-driven decision-making in diverse scientific disciplines.
Understanding Thermodynamics: Key Concepts
Thermodynamics explores energy transformations, encompassing concepts such as enthalpy, entropy, and Gibbs free energy to predict reaction spontaneity and system equilibrium. Statistical thermodynamics links microscopic particle behavior with macroscopic thermodynamic properties by using probability distributions and molecular states. Mastery of these principles enables accurate analysis of heat transfer, phase changes, and chemical reaction energetics in physical and chemical systems.
Historical Evolution of Statistical and Thermodynamic Theories
Statistical mechanics emerged in the late 19th century through the pioneering work of James Clerk Maxwell, Ludwig Boltzmann, and Josiah Willard Gibbs, providing a microscopic interpretation of thermodynamic phenomena via particle behavior and probability distributions. Thermodynamics, rooted in the early 19th century with contributions from Sadi Carnot, Rudolf Clausius, and Lord Kelvin, developed as a macroscopic theory describing energy, work, and heat without detailed insight into molecular dynamics. The historical evolution saw thermodynamics establish fundamental laws first, while statistical mechanics later offered a deeper theoretical foundation linking microscopic states to macroscopic properties.
Core Differences: Statistical vs Thermodynamic Perspectives
Statistical mechanics explains thermodynamic properties by analyzing the microscopic behavior of atoms and molecules using probability distributions, whereas thermodynamics deals with macroscopic states and equilibrium conditions without considering individual particles. Statistical approaches derive thermodynamic quantities like entropy and temperature from the underlying molecular configurations, while classical thermodynamics defines these quantities empirically based on bulk measurements. The core difference lies in statistical mechanics providing a microscopic foundation for thermodynamic laws through ensemble averages and fluctuations, contrasting with thermodynamics' phenomenological and macroscopic viewpoint.
Applications in Physics: Where They Intersect and Diverge
Statistical mechanics bridges microscopic particle behavior with macroscopic thermodynamic properties, providing a probabilistic framework essential for understanding phase transitions, heat capacities, and entropy in physical systems. Thermodynamics offers macroscopic laws governing energy, work, and efficiency without delving into particle-level details, making it crucial for engineering applications and engines. Their intersection lies in explaining equilibrium states, while they diverge in approach: statistical mechanics uses probability distributions and microstates, whereas thermodynamics relies on state variables and empirical laws.
Mathematical Frameworks: From Probability to Energy Laws
Statistical mechanics employs probability theory and combinatorics to connect microscopic particle states with macroscopic observables, framing thermodynamic quantities in terms of statistical distributions. Thermodynamics relies on energy laws such as the first and second laws, using state variables and empirical relationships to describe system behavior without detailing molecular configurations. The mathematical framework of statistical mechanics bridges individual particle probabilities with thermodynamic potentials, providing a rigorous foundation grounded in entropy, free energy, and partition functions.
Limitations and Strengths of Statistical Approaches
Statistical approaches excel in modeling systems with large numbers of particles by utilizing probability distributions and microscopic states, providing detailed insights into molecular behavior and fluctuations. Their limitations include computational intensity and challenges in accurately capturing long-range interactions or phase transitions in complex systems. Despite these constraints, statistical methods effectively bridge microscopic properties with macroscopic thermodynamic quantities, making them essential for understanding equilibrium and non-equilibrium phenomena.
Thermodynamic Laws: Bridging Macroscopic and Microscopic Views
Thermodynamic laws provide fundamental principles governing energy and entropy changes in macroscopic systems, bridging the gap between observable phenomena and microscopic particle behavior. The zeroth, first, second, and third laws offer a framework to predict system equilibrium, energy conservation, entropy increase, and absolute zero temperature states based on bulk properties. Statistical mechanics complements these laws by linking microscopic particle distributions and probabilistic states to macroscopic thermodynamic quantities, enabling a comprehensive understanding of physical systems.
Future Trends: Integrating Statistical and Thermodynamic Methods
Future trends in integrating statistical and thermodynamic methods emphasize the development of hybrid computational models that leverage machine learning algorithms to enhance predictive accuracy in material science and chemical engineering. Advances in big data analytics enable the extraction of complex patterns from thermodynamic datasets, improving the reliability of phase equilibrium and reaction kinetics predictions. Continued collaboration between statistics and thermodynamics promises to optimize energy systems, accelerate drug discovery, and refine climate modeling frameworks.
Statistical Infographic
 libterm.com
libterm.com