Catalysis accelerates chemical reactions by lowering activation energy, while photocatalysis uses light to activate a catalyst, enabling environmentally friendly processes like pollutant degradation and solar fuel production. Understanding these mechanisms can enhance your ability to harness them for industrial and environmental applications. Explore the rest of the article to discover how catalysis and photocatalysis are transforming modern technology.
Table of Comparison
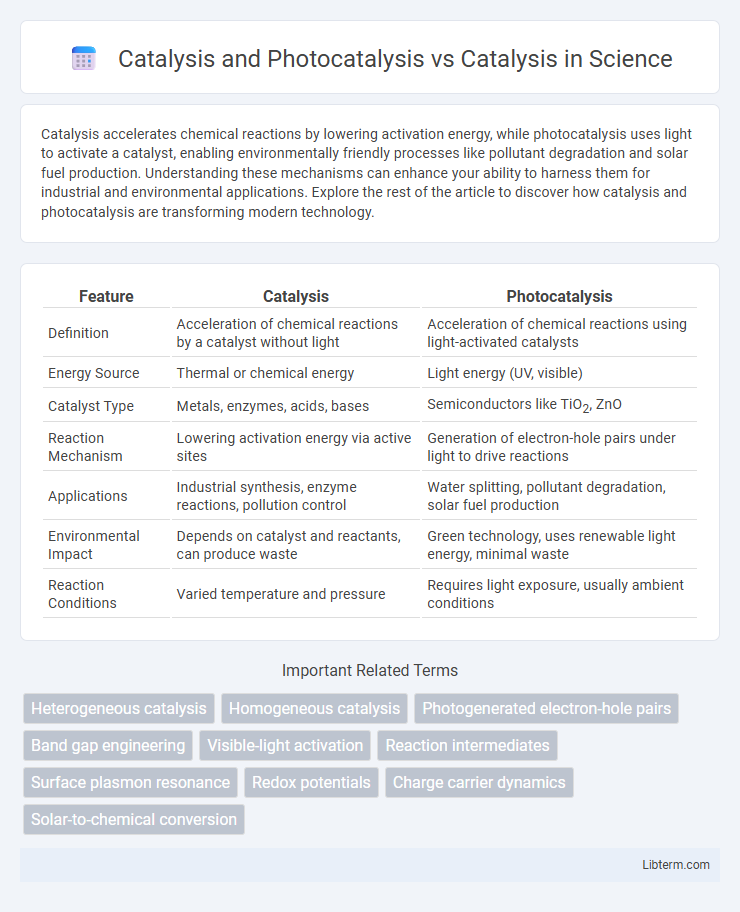
| Feature | Catalysis | Photocatalysis |
|---|---|---|
| Definition | Acceleration of chemical reactions by a catalyst without light | Acceleration of chemical reactions using light-activated catalysts |
| Energy Source | Thermal or chemical energy | Light energy (UV, visible) |
| Catalyst Type | Metals, enzymes, acids, bases | Semiconductors like TiO2, ZnO |
| Reaction Mechanism | Lowering activation energy via active sites | Generation of electron-hole pairs under light to drive reactions |
| Applications | Industrial synthesis, enzyme reactions, pollution control | Water splitting, pollutant degradation, solar fuel production |
| Environmental Impact | Depends on catalyst and reactants, can produce waste | Green technology, uses renewable light energy, minimal waste |
| Reaction Conditions | Varied temperature and pressure | Requires light exposure, usually ambient conditions |
Introduction to Catalysis and Photocatalysis
Catalysis accelerates chemical reactions by lowering activation energy through the use of catalysts, which remain unchanged after the reaction. Photocatalysis involves the activation of a catalyst by light, typically ultraviolet or visible radiation, enabling reactions under milder conditions and offering enhanced selectivity for processes like pollutant degradation and water splitting. Understanding the distinction between thermal catalysis and photocatalysis is crucial for optimizing applications in energy conversion and environmental remediation.
Defining Traditional Catalysis
Traditional catalysis involves substances called catalysts that increase the rate of chemical reactions without being consumed in the process, often working by lowering the activation energy. Catalysts in traditional catalysis can be homogeneous, dispersed in the same phase as reactants, or heterogeneous, existing in a different phase, such as solid catalysts in liquid or gas-phase reactions. This fundamental approach contrasts with photocatalysis, where light energy activates the catalyst to drive or enhance chemical transformations.
Fundamentals of Photocatalysis
Photocatalysis harnesses light energy to activate a catalyst, enabling chemical reactions that are otherwise thermodynamically unfavorable or slow under visible or UV light exposure. Unlike traditional catalysis, which relies solely on thermal energy to lower activation energy, photocatalysis involves electron-hole pair generation in semiconductor materials such as TiO2, facilitating redox reactions on catalyst surfaces. The fundamental mechanism of photocatalysis includes photon absorption, charge carrier separation, and surface interaction with reactants, making it highly effective for environmental remediation and solar fuel production.
Mechanistic Differences Between Catalysis and Photocatalysis
Catalysis involves the acceleration of chemical reactions through lowering activation energy by providing an alternative reaction pathway, typically via the interaction of reactants with active sites on a catalyst surface. Photocatalysis, however, relies on light absorption by the catalyst to generate electron-hole pairs, which drive redox reactions through charge transfer mechanisms distinct from thermal activation. Mechanistically, catalysis primarily depends on surface adsorption and transition state stabilization, whereas photocatalysis integrates photo-induced charge separation and migration processes, enabling unique reaction pathways influenced by photon energy and catalyst band structure.
Key Materials Used in Catalysis and Photocatalysis
Catalysis primarily utilizes metals such as platinum, palladium, and rhodium as key materials due to their high catalytic activity and selectivity in chemical transformations. Photocatalysis relies heavily on semiconductor materials like titanium dioxide (TiO2), zinc oxide (ZnO), and graphitic carbon nitride (g-C3N4) that can generate electron-hole pairs under light irradiation to drive reactions. Both fields emphasize surface properties and bandgap engineering to enhance efficiency, but photocatalysis uniquely integrates light absorption and charge separation for sustainable chemical processes.
Energy Sources: Thermal Versus Light Activation
Catalysis typically relies on thermal energy to activate reactants and lower activation energy, whereas photocatalysis utilizes light energy, often from visible or ultraviolet sources, to excite electrons and initiate chemical reactions. Photocatalysis enables energy-efficient pathways under mild conditions by harnessing photon-induced charge carriers, making it suitable for sustainable applications like solar fuel production and environmental remediation. Thermal catalysis, on the other hand, is widely used in industrial processes requiring high temperatures, such as hydrocarbon reforming and ammonia synthesis.
Efficiency and Selectivity Comparisons
Catalysis enhances reaction rates by lowering activation energy, while photocatalysis utilizes light energy to activate catalysts, often increasing efficiency under mild conditions. Photocatalysis demonstrates higher selectivity in oxidation reactions due to controlled electron-hole pair generation, reducing side products compared to traditional catalysis. Efficiency in photocatalysis depends on light absorption and charge separation, making it superior for eco-friendly processes like pollutant degradation and solar fuel production.
Environmental Impact and Sustainability Considerations
Catalysis and photocatalysis both play crucial roles in environmental impact reduction by enhancing reaction efficiency and enabling green chemical processes. Photocatalysis leverages solar energy to drive degradation of pollutants and wastewater treatment, offering a sustainable approach with minimal energy consumption and lower greenhouse gas emissions compared to traditional catalytic methods. The integration of photocatalytic systems promotes sustainability by utilizing renewable energy sources, reducing reliance on fossil fuels, and enabling more eco-friendly industrial practices.
Industrial and Practical Applications
Catalysis accelerates chemical reactions in industrial processes like petroleum refining, pharmaceutical synthesis, and polymer production, enhancing efficiency and selectivity. Photocatalysis harnesses light energy, enabling sustainable applications such as water purification, air filtration, and solar fuel production by driving reactions under mild conditions. Industrial adoption of photocatalysts focuses on environmental remediation and green chemistry, complementing traditional catalysis by reducing energy consumption and minimizing hazardous byproducts.
Future Trends in Catalysis and Photocatalysis
Future trends in catalysis emphasize the development of sustainable and energy-efficient processes, integrating renewable feedstocks and reducing environmental impact. Advances in photocatalysis focus on harnessing solar energy to drive chemical reactions, enabling greener synthetic routes and decomposition of pollutants under mild conditions. Emerging materials such as metal-organic frameworks (MOFs) and plasmonic nanoparticles enhance catalytic efficiency and selectivity, shaping the future of both traditional catalysis and photocatalysis applications.
Catalysis and Photocatalysis Infographic
 libterm.com
libterm.com