Autophagy is a vital cellular process that helps maintain homeostasis by degrading and recycling damaged organelles and proteins, ensuring cell survival during stress. This mechanism plays a crucial role in preventing diseases such as cancer, neurodegeneration, and infections by regulating cell health and renewal. Discover how autophagy influences your body's well-being and the latest insights into harnessing this process in the full article.
Table of Comparison
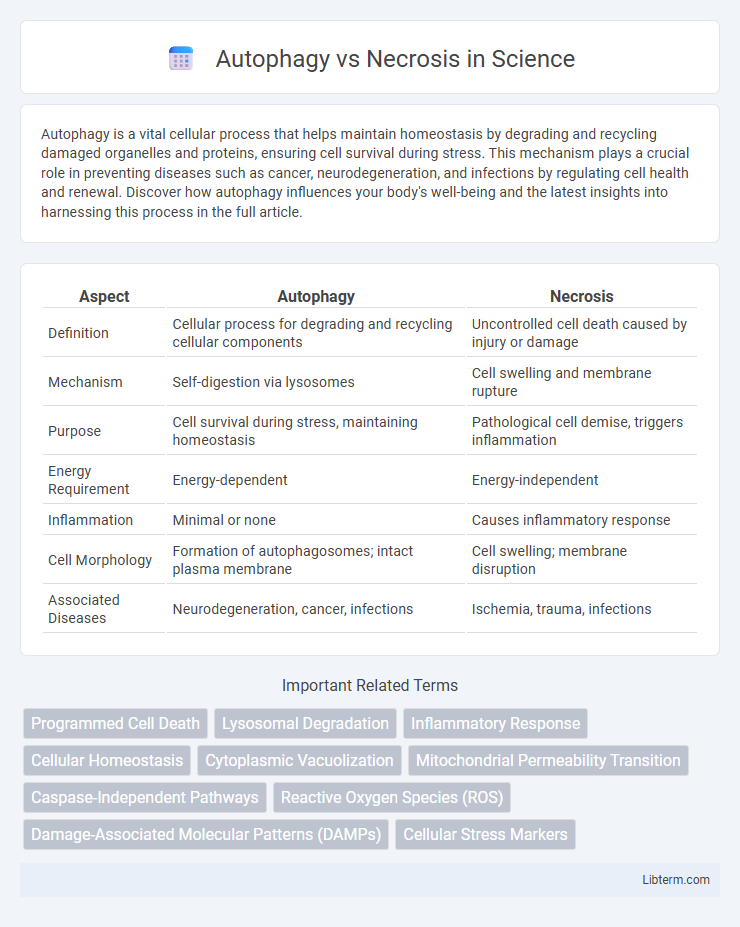
| Aspect | Autophagy | Necrosis |
|---|---|---|
| Definition | Cellular process for degrading and recycling cellular components | Uncontrolled cell death caused by injury or damage |
| Mechanism | Self-digestion via lysosomes | Cell swelling and membrane rupture |
| Purpose | Cell survival during stress, maintaining homeostasis | Pathological cell demise, triggers inflammation |
| Energy Requirement | Energy-dependent | Energy-independent |
| Inflammation | Minimal or none | Causes inflammatory response |
| Cell Morphology | Formation of autophagosomes; intact plasma membrane | Cell swelling; membrane disruption |
| Associated Diseases | Neurodegeneration, cancer, infections | Ischemia, trauma, infections |
Introduction to Autophagy and Necrosis
Autophagy is a cellular degradation process where cells recycle damaged organelles and proteins through lysosomal machinery, promoting survival under stress conditions. Necrosis, in contrast, is an uncontrolled form of cell death characterized by membrane rupture and inflammation, often resulting from acute injury. Understanding the molecular mechanisms distinguishing autophagy and necrosis is critical in disease pathogenesis and therapeutic development.
Defining Autophagy: The Cellular Recycling Process
Autophagy is a critical cellular recycling process where damaged organelles and misfolded proteins are engulfed by autophagosomes and degraded for nutrient reuse, maintaining cellular homeostasis. In contrast to necrosis, which is an uncontrolled form of cell death causing inflammation and tissue damage, autophagy is a regulated mechanism promoting cell survival under stress by removing dysfunctional components. Understanding autophagy's role in diseases like cancer and neurodegeneration highlights its importance in therapeutic strategies targeting cellular maintenance and longevity.
Understanding Necrosis: Accidental Cell Death
Necrosis is a form of accidental cell death characterized by the uncontrolled rupture of the cell membrane, leading to the release of cellular contents and inflammation in surrounding tissues. Unlike autophagy, which is a regulated process for recycling damaged organelles and proteins, necrosis results from acute cellular injury such as trauma, toxins, or ischemia. This uncontrolled cell death contributes to pathological conditions including infection, neurodegeneration, and tissue damage after injury.
Key Molecular Mechanisms Behind Autophagy
Autophagy is regulated by key molecular mechanisms including the ULK1 complex that initiates phagophore formation and the mTOR pathway which inhibits autophagy under nutrient-rich conditions. The process also involves LC3 conjugation to membranes for autophagosome maturation and ATG proteins facilitating vesicle expansion and cargo sequestration. Unlike necrosis, which is characterized by uncontrolled cell membrane rupture and inflammation, autophagy maintains cellular homeostasis through selective lysosomal degradation of damaged organelles and proteins.
Cellular Pathways Involved in Necrosis
Necrosis involves unregulated cell death characterized by cellular swelling, plasma membrane rupture, and inflammation, primarily triggered by external insults like toxins or ischemia. Key cellular pathways include the activation of receptor-interacting protein kinase 1 and 3 (RIPK1/RIPK3) leading to necroptosis, disruption of mitochondrial function causing ATP depletion, and excessive calcium influx that activates degradative enzymes. These pathways result in loss of membrane integrity and uncontrolled release of intracellular components, distinguishing necrosis from the regulated process of autophagy.
Autophagy vs Necrosis: Major Differences
Autophagy is a regulated cellular process that degrades and recycles damaged organelles and proteins to maintain cell homeostasis, whereas necrosis is an uncontrolled form of cell death resulting from acute cellular injury leading to cell membrane rupture and inflammation. Autophagy involves lysosomal degradation pathways promoting cell survival under stress, while necrosis causes cellular swelling, membrane rupture, and release of intracellular contents that trigger immune responses. Key molecular markers such as LC3-II indicate autophagy activation, whereas necrosis is characterized by the loss of plasma membrane integrity and elevated levels of damage-associated molecular patterns (DAMPs).
Triggers and Causes of Autophagy and Necrosis
Autophagy is triggered by nutrient deprivation, cellular stress, or damaged organelles, initiating a regulated process to recycle cellular components and maintain homeostasis. Necrosis occurs primarily due to acute injury, toxins, or ischemia, leading to uncontrolled cell membrane rupture and inflammation. While autophagy promotes cell survival through lysosomal degradation, necrosis results in cell death and tissue damage.
Impact on Tissue Homeostasis and Disease
Autophagy plays a crucial role in maintaining tissue homeostasis by recycling damaged cellular components, thereby preventing the accumulation of toxic aggregates and supporting cell survival under stress conditions. In contrast, necrosis, characterized by uncontrolled cell death and membrane rupture, causes inflammation and disrupts tissue architecture, often exacerbating disease progression. Dysregulated autophagy is implicated in neurodegenerative diseases and cancer, while necrosis contributes to acute tissue injury and chronic inflammatory disorders.
Clinical Implications: Therapeutic Perspectives
Autophagy enhances cellular homeostasis by degrading damaged organelles and proteins, offering potential therapeutic strategies for neurodegenerative diseases, cancer, and metabolic disorders. Necrosis, characterized by uncontrolled cell death and inflammation, often exacerbates tissue damage, making its inhibition a target for reducing injury in conditions like myocardial infarction and stroke. Modulating autophagy pathways through pharmacological agents or gene therapy presents promising clinical interventions to promote cell survival, while controlling necrosis limits inflammatory responses and tissue degeneration.
Conclusion: Balancing Cell Survival and Death
Autophagy promotes cell survival by degrading damaged organelles and proteins, maintaining cellular homeostasis under stress conditions. Necrosis results in uncontrolled cell death, often causing inflammation and tissue damage. Balancing autophagy and necrosis is crucial for regulating cell fate, preventing disease progression, and promoting tissue health.
Autophagy Infographic
 libterm.com
libterm.com