A nucleophile is a chemical species that donates an electron pair to form a chemical bond during a reaction, playing a crucial role in many organic and inorganic processes. These molecules or ions are characterized by their electron-rich nature, enabling them to attack positively charged or electron-deficient sites. Discover how nucleophiles influence reaction mechanisms and your understanding of chemical interactions by reading the rest of the article.
Table of Comparison
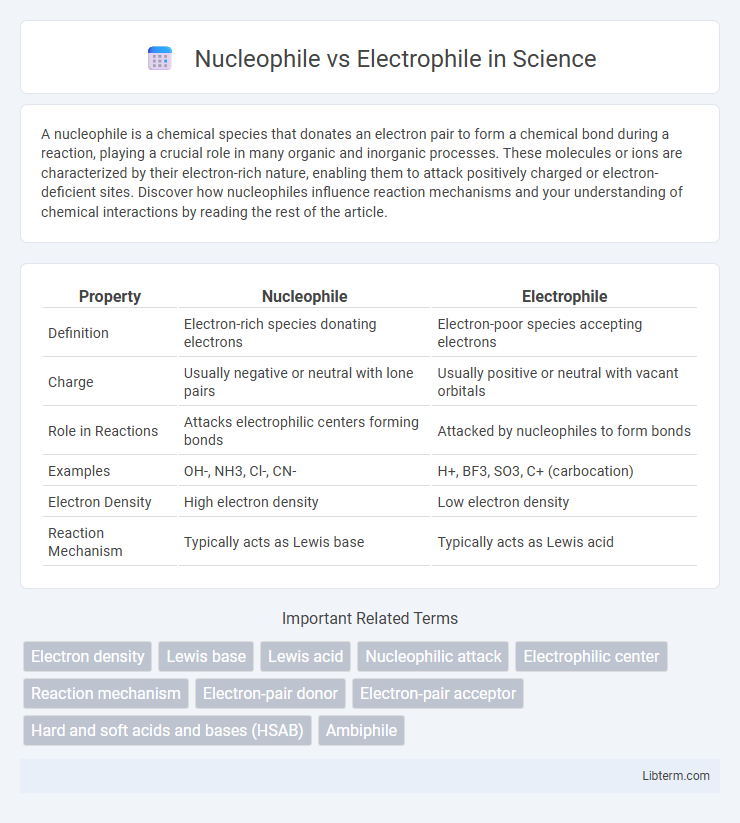
| Property | Nucleophile | Electrophile |
|---|---|---|
| Definition | Electron-rich species donating electrons | Electron-poor species accepting electrons |
| Charge | Usually negative or neutral with lone pairs | Usually positive or neutral with vacant orbitals |
| Role in Reactions | Attacks electrophilic centers forming bonds | Attacked by nucleophiles to form bonds |
| Examples | OH-, NH3, Cl-, CN- | H+, BF3, SO3, C+ (carbocation) |
| Electron Density | High electron density | Low electron density |
| Reaction Mechanism | Typically acts as Lewis base | Typically acts as Lewis acid |
Introduction to Nucleophiles and Electrophiles
Nucleophiles are chemical species characterized by their electron-rich nature, enabling them to donate an electron pair to electrophiles during chemical reactions. Electrophiles, on the other hand, possess an electron-deficient center and seek to accept electron pairs from nucleophiles to form new chemical bonds. Understanding the behavior of nucleophiles and electrophiles is fundamental in predicting reaction mechanisms in organic and inorganic chemistry.
Definition of Nucleophiles
Nucleophiles are chemical species that donate an electron pair to form a covalent bond, typically possessing lone pairs or p electrons that seek positively charged or electron-deficient centers. They play a crucial role in organic reactions such as nucleophilic substitution and addition by attacking electrophilic atoms or molecules. Common examples of nucleophiles include hydroxide ions (OH-), amines (NH3), and halide ions (Cl-, Br-).
Definition of Electrophiles
Electrophiles are chemical species that seek electrons to form bonds by accepting an electron pair from nucleophiles. They are typically positively charged or electron-deficient atoms or molecules, such as carbocations, carbonyl groups, and protonated species. Electrophiles play a crucial role in organic reactions by attacking nucleophiles, facilitating bond formation and molecular transformation.
Key Differences Between Nucleophiles and Electrophiles
Nucleophiles are electron-rich species that donate an electron pair to form a chemical bond, while electrophiles are electron-deficient species that accept electron pairs. Nucleophiles often possess lone pairs or p electrons, whereas electrophiles have positive charges, partial positive charges, or vacant orbitals. The reactivity of nucleophiles and electrophiles is governed by their electron density, polarity, and the nature of the reacting molecules.
Characteristics of Nucleophiles
Nucleophiles are characterized by their electron-rich nature, possessing lone pairs or p electrons that they donate during chemical reactions. They exhibit high electron density, which enables them to attack positively charged or electron-deficient electrophilic centers. Common nucleophiles include species such as hydroxide ion (OH-), amines (NH3), and halide ions (Cl-, Br-), all of which demonstrate strong nucleophilicity based on factors like charge, electronegativity, and solvent effects.
Characteristics of Electrophiles
Electrophiles are electron-deficient species that seek electron-rich centers to form covalent bonds, often possessing positive charges or partial positive charges due to electronegativity differences. Common electrophiles include carbocations, carbonyl compounds, and molecules with polarized bonds, making them highly reactive towards nucleophiles. Their key characteristic is the ability to accept electron pairs, facilitating reactions such as nucleophilic additions and substitutions in organic chemistry.
Common Examples of Nucleophiles
Common examples of nucleophiles include hydroxide ions (OH-), alkoxide ions (RO-), cyanide ions (CN-), ammonia (NH3), and halide ions such as chloride (Cl-), bromide (Br-), and iodide (I-). These species donate electron pairs to electrophiles during chemical reactions, typically attacking positively charged or electron-deficient centers. Nucleophiles play a crucial role in substitution and addition reactions in organic and inorganic chemistry.
Common Examples of Electrophiles
Electrophiles are electron-deficient species that seek to accept electron pairs from nucleophiles during chemical reactions, commonly found in organic and inorganic chemistry. Typical examples of electrophiles include carbocations like tert-butyl cation, acyl chlorides such as acetyl chloride, and molecules containing electron-withdrawing groups like carbonyl compounds (aldehydes and ketones). These electrophiles play crucial roles in substitution and addition reactions by interacting with nucleophiles to form new chemical bonds.
Nucleophilic and Electrophilic Reactions
Nucleophilic reactions involve nucleophiles, which are electron-rich species donating electron pairs to electrophilic centers with positive or partial positive charge, facilitating bond formation. Electrophilic reactions occur when electrophiles, electron-deficient species, accept electron pairs from nucleophiles or electron-rich sites, resulting in the creation of new chemical bonds. Understanding the distinct roles of nucleophiles and electrophiles in these reactions is essential for predicting reaction mechanisms in organic chemistry, including substitution, addition, and aromatic electrophilic substitution processes.
Importance in Organic Chemistry
Nucleophiles and electrophiles are essential in organic chemistry as they dictate the mechanisms of many reactions, including substitution and addition processes. Nucleophiles, rich in electron density, donate electron pairs to electrophiles, which are electron-deficient species, facilitating bond formation and molecular transformations. Understanding the behavior of nucleophiles and electrophiles enables chemists to predict reaction outcomes and design synthetic pathways efficiently.
Nucleophile Infographic
 libterm.com
libterm.com