Isomers are molecules with the same molecular formula but different arrangements of atoms, leading to distinct chemical properties, while enantiomers are a specific type of stereoisomer that are non-superimposable mirror images of each other. Understanding the subtle differences between isomers and enantiomers is crucial for fields such as pharmaceuticals, where the biological activity of each can vary dramatically. Explore the rest of this article to discover how these molecular differences impact your work or studies in chemistry.
Table of Comparison
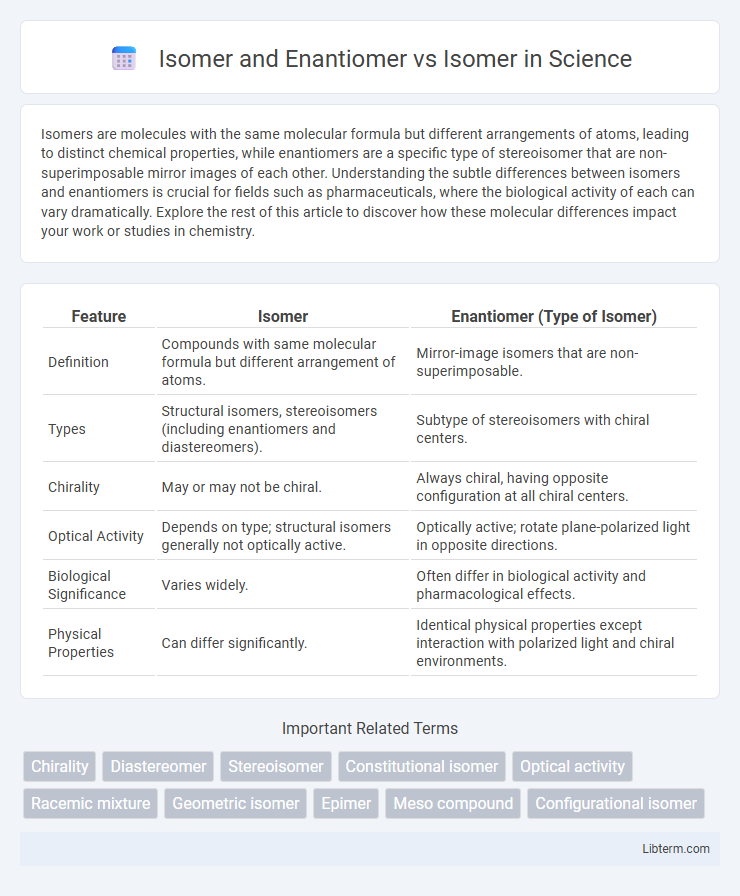
| Feature | Isomer | Enantiomer (Type of Isomer) |
|---|---|---|
| Definition | Compounds with same molecular formula but different arrangement of atoms. | Mirror-image isomers that are non-superimposable. |
| Types | Structural isomers, stereoisomers (including enantiomers and diastereomers). | Subtype of stereoisomers with chiral centers. |
| Chirality | May or may not be chiral. | Always chiral, having opposite configuration at all chiral centers. |
| Optical Activity | Depends on type; structural isomers generally not optically active. | Optically active; rotate plane-polarized light in opposite directions. |
| Biological Significance | Varies widely. | Often differ in biological activity and pharmacological effects. |
| Physical Properties | Can differ significantly. | Identical physical properties except interaction with polarized light and chiral environments. |
Introduction to Isomers
Isomers are molecules with the same molecular formula but different arrangements of atoms, leading to distinct physical and chemical properties. Isomers are broadly classified into structural isomers, which differ in connectivity, and stereoisomers, which have the same connectivity but differ in spatial arrangement; enantiomers are a specific type of stereoisomer that are non-superimposable mirror images of each other. Understanding isomers is fundamental in fields like organic chemistry and pharmaceuticals due to their impact on molecular function and biological activity.
Types of Isomers: An Overview
Isomers are compounds with the same molecular formula but different structures, and they are primarily categorized into structural isomers and stereoisomers. Structural isomers differ in the arrangement of atoms, while stereoisomers share the same atom connectivity but vary in spatial orientation. Enantiomers, a key type of stereoisomers, are non-superimposable mirror images of each other, crucial in fields like pharmaceuticals where chirality affects drug effectiveness and safety.
What Are Enantiomers?
Enantiomers are a type of stereoisomer that are non-superimposable mirror images of each other, differing in the spatial arrangement around a chiral center. Unlike general isomers, which include structural isomers differing in connectivity, enantiomers share the same molecular formula and sequence of bonded atoms but exhibit different optical activities. This chiral distinction makes enantiomers particularly important in fields like pharmaceuticals, where they can have drastically different biological effects.
Isomer vs Enantiomer: Key Differences
Isomers are molecules with the same molecular formula but different structural arrangements, while enantiomers are a specific type of stereoisomer that are non-superimposable mirror images of each other. The key differences between isomers and enantiomers lie in their spatial configuration and optical activity, with enantiomers exhibiting chirality and rotating plane-polarized light in opposite directions. Understanding these distinctions is crucial in fields such as pharmaceutical chemistry, where enantiomeric purity can impact drug efficacy and safety.
Structural Isomers vs Stereoisomers
Structural isomers differ in the connectivity of atoms, resulting in distinct molecular frameworks, while stereoisomers share the same atomic connectivity but differ in the spatial arrangement of atoms. Among stereoisomers, enantiomers are non-superimposable mirror images, exhibiting chirality, whereas diastereomers are stereoisomers that are not mirror images. The distinction between structural isomers and stereoisomers is critical in chemical properties and biological activity, especially in pharmaceuticals and materials science.
Importance of Enantiomers in Chemistry
Enantiomers are a specific type of isomer characterized by non-superimposable mirror images, playing a crucial role in stereochemistry due to their impact on molecular interactions and biological activity. Unlike general isomers, which differ in connectivity or spatial arrangement, enantiomers exhibit identical physical properties but differ markedly in optical rotation and reactivity with chiral environments. The importance of enantiomers in chemistry is emphasized by their distinct effects in pharmaceuticals, where one enantiomer may be therapeutic while the other is inactive or harmful, making chiral purity essential in drug design and synthesis.
Biological Significance of Enantiomers
Enantiomers exhibit distinct biological significance due to their chiral nature, affecting drug efficacy and metabolic pathways by interacting differently with enzymes and receptors. Unlike general isomers, which differ in atomic arrangement without necessarily altering biological activity, enantiomers have mirror-image structures essential for stereospecific processes in living organisms. This stereochemistry influences pharmacodynamics and pharmacokinetics, making enantiomeric purity critical in pharmaceutical development.
Methods for Identifying Isomers and Enantiomers
Techniques for identifying isomers and enantiomers rely on their distinct structural and optical properties. Nuclear Magnetic Resonance (NMR) spectroscopy differentiates isomers by analyzing unique chemical environments, while chiral chromatography separates enantiomers based on their interaction with chiral stationary phases. Optical rotation measurement using polarimetry specifically identifies enantiomers due to their ability to rotate plane-polarized light in opposite directions.
Real-World Applications of Isomers and Enantiomers
Isomers and enantiomers play crucial roles in pharmaceuticals, with enantiomers often exhibiting drastically different biological activities, influencing drug efficacy and safety. Isomers are vital in materials science, where structural variations affect polymer properties and performance. The pharmaceutical, agrochemical, and food industries rely on stereoselective synthesis of enantiomers to optimize product effectiveness and regulatory compliance.
Summary: Isomer and Enantiomer Differences
Isomers are compounds with the same molecular formula but different structural arrangements, while enantiomers are a specific type of stereoisomer that are non-superimposable mirror images. Isomers include structural isomers and stereoisomers, but enantiomers are characterized by their chiral centers and optical activity. The key difference lies in that all enantiomers are isomers, but not all isomers are enantiomers.
Isomer and Enantiomer Infographic
 libterm.com
libterm.com