Isothermal processes maintain constant temperature throughout, ensuring energy exchange occurs without a change in thermal state. This principle is crucial in thermodynamics, widely applied in engines, refrigerators, and climate control systems to optimize efficiency. Discover how isothermal conditions influence your daily technologies by exploring the rest of the article.
Table of Comparison
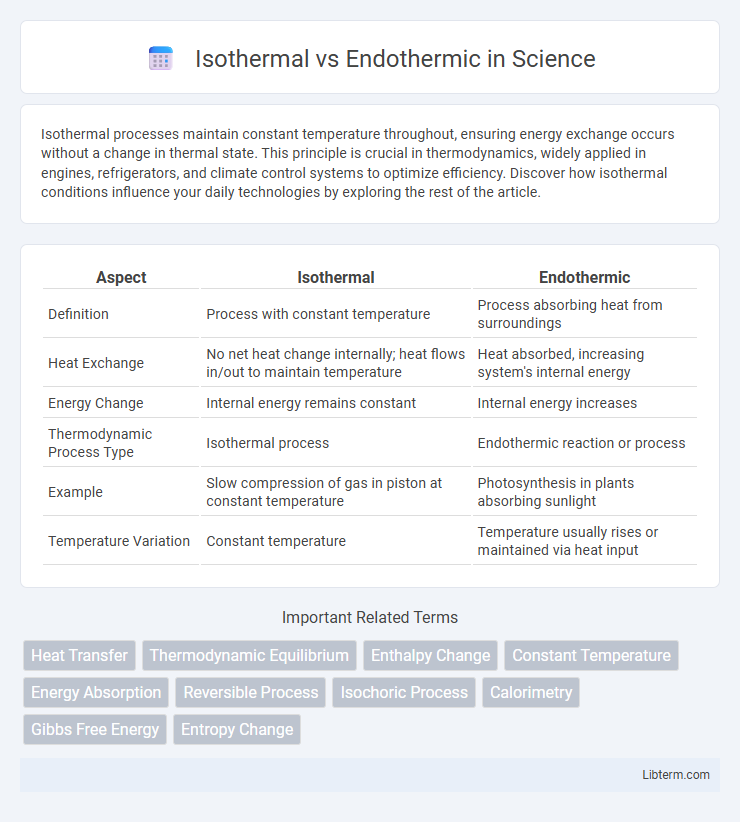
| Aspect | Isothermal | Endothermic |
|---|---|---|
| Definition | Process with constant temperature | Process absorbing heat from surroundings |
| Heat Exchange | No net heat change internally; heat flows in/out to maintain temperature | Heat absorbed, increasing system's internal energy |
| Energy Change | Internal energy remains constant | Internal energy increases |
| Thermodynamic Process Type | Isothermal process | Endothermic reaction or process |
| Example | Slow compression of gas in piston at constant temperature | Photosynthesis in plants absorbing sunlight |
| Temperature Variation | Constant temperature | Temperature usually rises or maintained via heat input |
Introduction to Isothermal and Endothermic Processes
Isothermal processes occur at a constant temperature, where heat exchange with the surroundings maintains thermal equilibrium, often seen in ideal gas expansions or compressions. Endothermic processes absorb heat from the environment, causing a rise in internal energy or phase changes such as melting or evaporation. Understanding the distinction between isothermal and endothermic reactions is crucial for applications in thermodynamics and chemical engineering.
Defining Isothermal Processes
Isothermal processes occur at a constant temperature, where the system exchanges heat with its surroundings to maintain thermal equilibrium. In contrast, endothermic processes absorb heat, resulting in an increase in the system's internal energy and often a rise in temperature. Understanding isothermal processes is critical for applications involving phase changes and ideal gas behavior under steady thermal conditions.
Understanding Endothermic Processes
Endothermic processes absorb heat energy from their surroundings, causing a temperature decrease in the environment. Unlike isothermal processes, where temperature remains constant, endothermic reactions require continuous energy input to proceed, such as melting ice or photosynthesis. Understanding the heat absorption characteristic is crucial in fields like thermodynamics and chemical engineering for designing energy-efficient systems.
Key Differences Between Isothermal and Endothermic
Isothermal processes maintain a constant temperature by exchanging heat with the surroundings, whereas endothermic reactions absorb heat, causing a temperature increase in the system. Isothermal processes focus on energy transfer without changing internal energy, while endothermic reactions involve an absorption of heat leading to an increase in enthalpy. The key difference lies in temperature stability during isothermal changes versus energy intake and temperature changes in endothermic reactions.
Thermodynamic Principles in Each Process
Isothermal processes maintain constant temperature by allowing heat exchange with the surroundings, ensuring the internal energy of the system remains unchanged according to the first law of thermodynamics. Endothermic reactions absorb heat, increasing the system's internal energy and often resulting in temperature changes unless heat is supplied continuously. Thermodynamically, isothermal processes are characterized by zero change in internal energy (DU = 0), while endothermic processes involve positive enthalpy change (DH > 0), reflecting heat absorption from the environment.
Real-World Examples of Isothermal Reactions
Isothermal reactions maintain a constant temperature while reacting, commonly seen in the dissolution of salt in water and enzyme-catalyzed biochemical processes where heat exchange with the environment balances any thermal changes. Endothermic reactions absorb heat, raising the system's temperature, exemplified by the melting of ice or the evaporation of water. Practical applications of isothermal reactions include industrial fermentation and biochemical assays that rely on steady temperatures to ensure reaction efficiency and stability.
Common Endothermic Reaction Scenarios
Common endothermic reaction scenarios include photosynthesis, where plants absorb sunlight to convert carbon dioxide and water into glucose and oxygen, and the melting of ice, which requires heat absorption to change solid ice into liquid water. Another typical example is the thermal decomposition of calcium carbonate in cement production, necessitating significant heat intake to break chemical bonds. These processes exemplify endothermic reactions by consistently requiring external energy input to proceed.
Energy Transfer: Isothermal vs Endothermic
Isothermal processes maintain constant temperature by allowing heat transfer between the system and surroundings, ensuring energy input or output balances work done without changing internal energy. Endothermic reactions absorb heat from the environment, increasing system energy and causing a temperature rise or requiring continuous heat input to sustain the process. The key difference lies in isothermal processes regulating energy flow to keep temperature stable, while endothermic processes depend on energy absorption to drive chemical or physical changes.
Applications in Industry and Science
Isothermal processes, maintaining constant temperature, are crucial in industries like chemical manufacturing and fermentation to ensure reaction stability and product consistency. Endothermic reactions, which absorb heat, are essential in processes such as thermal decomposition in metallurgy and photosynthesis research to study energy transfer and material properties. Both applications leverage temperature control to optimize reaction efficiency and product yield in scientific experiments and industrial production.
Summary: Choosing the Right Process
Isothermal processes maintain a constant temperature by allowing heat exchange with the surroundings, ideal for reactions requiring thermal stability. Endothermic processes absorb heat, increasing system temperature and driving reactions that need energy input. Selecting the right process depends on reaction type, energy efficiency, and desired temperature control for optimal reaction outcomes.
Isothermal Infographic
 libterm.com
libterm.com