Mastering basic skills lays a strong foundation for your personal and professional growth, empowering you to tackle more complex challenges with confidence. Understanding essential concepts enhances problem-solving abilities and fosters effective communication. Explore the full article to discover practical tips and strategies to strengthen your basic knowledge.
Table of Comparison
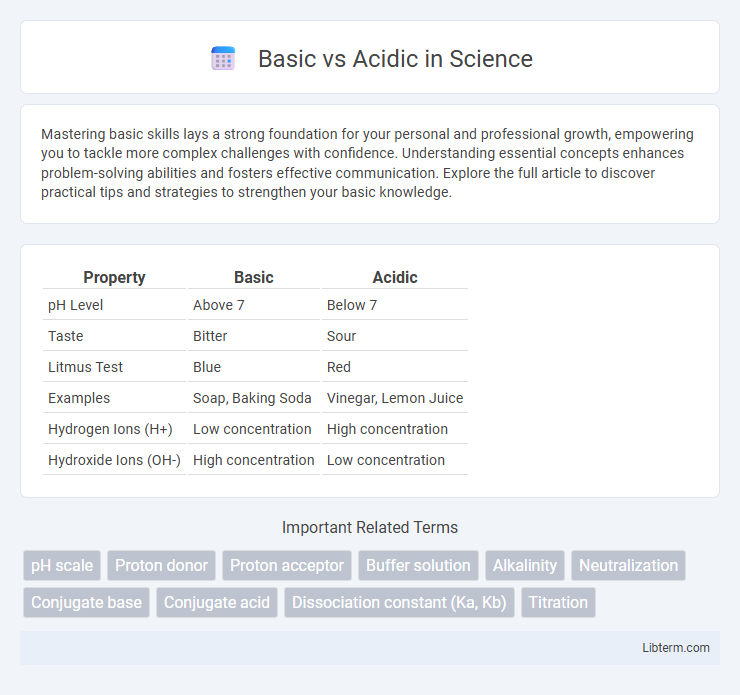
| Property | Basic | Acidic |
|---|---|---|
| pH Level | Above 7 | Below 7 |
| Taste | Bitter | Sour |
| Litmus Test | Blue | Red |
| Examples | Soap, Baking Soda | Vinegar, Lemon Juice |
| Hydrogen Ions (H+) | Low concentration | High concentration |
| Hydroxide Ions (OH-) | High concentration | Low concentration |
Understanding the Basics: What Are Acids and Bases?
Acids are substances that release hydrogen ions (H+) in aqueous solutions, resulting in a pH less than 7, while bases release hydroxide ions (OH-) and typically have a pH greater than 7. Common acids include hydrochloric acid (HCl) and sulfuric acid (H2SO4), whereas sodium hydroxide (NaOH) and ammonia (NH3) are typical bases. Understanding the pH scale, which ranges from 0 (strongly acidic) to 14 (strongly basic), is essential for differentiating between acidic and basic substances and their chemical behaviors.
Key Differences Between Acidic and Basic Substances
Acidic substances have a pH less than 7, release hydrogen ions (H+) in aqueous solutions, and taste sour, whereas basic substances have a pH greater than 7, release hydroxide ions (OH-), and taste bitter or feel slippery. Acids corrode metals and turn blue litmus paper red, while bases neutralize acids and turn red litmus paper blue. Common examples of acids include hydrochloric acid (HCl) and citric acid, while sodium hydroxide (NaOH) and ammonia (NH3) are typical bases.
The pH Scale Explained: Measuring Acidity and Basicity
The pH scale measures acidity and basicity on a range from 0 to 14, where values below 7 indicate acidic solutions and values above 7 indicate basic (alkaline) solutions. A pH of 7 is considered neutral, representing pure water with equal hydrogen ion (H+) and hydroxide ion (OH-) concentrations. Understanding pH is essential for applications in chemistry, biology, agriculture, and environmental science, as it directly influences chemical reactions, biological processes, and soil health.
Common Examples of Acidic and Basic Solutions
Common acidic solutions include lemon juice, vinegar, and stomach acid, all exhibiting a pH below 7 due to high hydrogen ion concentration. Basic solutions such as baking soda dissolved in water, ammonia cleaners, and soap solutions have a pH above 7, resulting from increased hydroxide ions. These examples highlight the fundamental distinction between acids and bases in everyday substances and their impact on chemical reactions and biological processes.
How Acids and Bases Affect Everyday Life
Acids, such as lemon juice and vinegar, influence everyday life by providing sour flavors and acting as natural preservatives, while bases like baking soda and soap are essential for cleaning and neutralizing odors. The pH balance of water and soil, influenced by acidic or basic substances, affects plant growth and water quality. Human health also depends on maintaining proper acid-base balance, as imbalances can lead to conditions like acid reflux or alkalosis.
Chemical Properties: Reactions of Acids vs. Bases
Acids release hydrogen ions (H+) in aqueous solutions, while bases release hydroxide ions (OH-), leading to distinct chemical behaviors. Acidic reactions typically involve proton donation and neutralization with bases to form water and salts. Bases engage in proton acceptance and react with acids, metals, or organic compounds, demonstrating unique reactivity patterns in chemical processes.
Health Impacts: Acidic vs. Basic Foods and Diets
Consuming a balanced diet with appropriate pH levels can significantly impact overall health, as excessively acidic diets are linked to increased risks of inflammation, kidney stones, and bone density loss. Alkaline or basic foods, such as fruits, vegetables, and nuts, help neutralize excess acid in the body, promoting better cellular function and reducing the likelihood of chronic diseases. Monitoring the acid-base balance through diet optimizes metabolic processes and supports long-term wellness.
Industrial and Household Uses of Acids and Bases
Industrial applications of acids include manufacturing fertilizers, refining metals, and producing batteries, while bases are essential for soap and detergent production, textile processing, and water treatment. Household uses of acids involve cleaning agents like vinegar for descaling and lemon juice for stain removal, whereas bases such as baking soda are used for odor neutralization and grease cutting. Both acids and bases play crucial roles in chemical reactions, sanitation, and maintenance across industrial and domestic environments.
Environmental Effects of Acids and Bases
Acids, such as sulfuric acid from industrial emissions, contribute to environmental acidification, leading to soil nutrient depletion and aquatic life harm. Bases, like ammonia from agricultural runoff, can increase water alkalinity, disrupting aquatic ecosystems and reducing biodiversity. Monitoring pH levels in soil and water is critical for assessing the environmental impact of acidic and basic substances.
Safety Tips When Handling Acidic and Basic Materials
Handling acidic and basic materials requires careful safety measures to prevent chemical burns and harmful reactions. Always wear appropriate personal protective equipment such as gloves, goggles, and lab coats, and ensure proper ventilation in the workspace to avoid inhaling fumes. Store acids and bases separately in clearly labeled, corrosion-resistant containers to minimize the risk of dangerous mixing or spills.
Basic Infographic
 libterm.com
libterm.com