Humanized experiences create deeper emotional connections by making interactions feel more personal and relatable. Brands that emphasize empathy and authenticity often build stronger trust and loyalty among their audiences. Discover how humanized approaches can transform Your engagement in the full article.
Table of Comparison
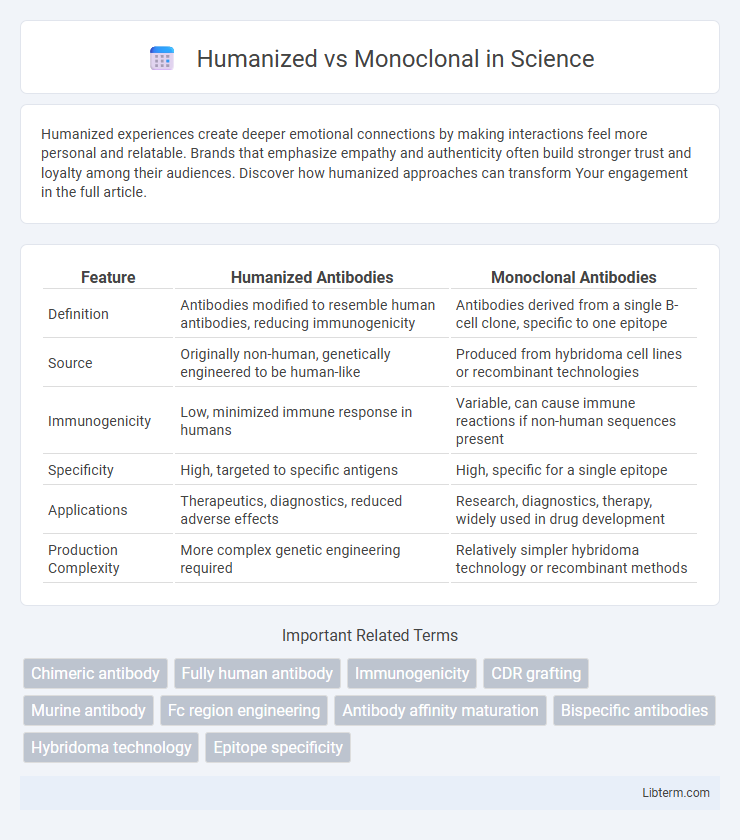
| Feature | Humanized Antibodies | Monoclonal Antibodies |
|---|---|---|
| Definition | Antibodies modified to resemble human antibodies, reducing immunogenicity | Antibodies derived from a single B-cell clone, specific to one epitope |
| Source | Originally non-human, genetically engineered to be human-like | Produced from hybridoma cell lines or recombinant technologies |
| Immunogenicity | Low, minimized immune response in humans | Variable, can cause immune reactions if non-human sequences present |
| Specificity | High, targeted to specific antigens | High, specific for a single epitope |
| Applications | Therapeutics, diagnostics, reduced adverse effects | Research, diagnostics, therapy, widely used in drug development |
| Production Complexity | More complex genetic engineering required | Relatively simpler hybridoma technology or recombinant methods |
Introduction to Therapeutic Antibodies
Therapeutic antibodies are engineered proteins designed to target specific antigens involved in disease processes, offering precision treatment options. Humanized antibodies are derived from non-human species but modified to closely resemble human antibodies, reducing immunogenicity while maintaining antigen specificity. Monoclonal antibodies originate from a single immune cell clone, ensuring uniformity and high specificity, but can be fully human, humanized, chimeric, or murine depending on their source and engineering.
Defining Monoclonal Antibodies
Monoclonal antibodies are laboratory-produced molecules engineered to serve as substitute antibodies that can restore, enhance, or mimic the immune system's attack on harmful cells. These antibodies are designed from a single clone of B cells, ensuring specificity to a singular antigen epitope, which makes them highly effective in targeting cancers, autoimmune diseases, and infections. In contrast, humanized antibodies are modified monoclonal antibodies with reduced immunogenicity, achieved by grafting non-human antigen-binding sites onto human antibody frameworks.
What Are Humanized Antibodies?
Humanized antibodies are engineered proteins that closely resemble natural human antibodies, designed by grafting non-human antibody variable regions onto human antibody frameworks to reduce immune rejection in therapeutic applications. Unlike fully monoclonal antibodies derived solely from a single clone, humanized antibodies minimize immunogenicity while maintaining high specificity and affinity for target antigens. These antibodies play a crucial role in improving the safety and efficacy of treatments for cancer, autoimmune diseases, and infectious conditions.
Development Process: Monoclonal vs Humanized
Monoclonal antibodies are developed by fusing a specific B-cell with a myeloma cell to create a hybridoma that produces identical antibodies targeting a single epitope. Humanized antibodies undergo genetic engineering to replace most of the mouse antibody structure with human antibody sequences, retaining only the antigen-binding sites to reduce immunogenicity. This process involves advanced recombinant DNA techniques and molecular cloning to optimize antibody compatibility with the human immune system.
Structural Differences and Similarities
Humanized and monoclonal antibodies share a Y-shaped structure composed of two heavy and two light chains, allowing antigen binding via variable regions. Humanized antibodies have mouse-derived complementarity-determining regions (CDRs) grafted onto a human antibody framework, reducing immunogenicity compared to fully murine monoclonal antibodies. Both types retain high specificity for target antigens, but humanized antibodies exhibit greater compatibility for therapeutic use in humans due to their structural composition.
Clinical Applications and Effectiveness
Humanized antibodies exhibit reduced immunogenicity compared to murine monoclonal antibodies, enhancing clinical tolerability and allowing repeated dosing in therapies such as cancer and autoimmune diseases. Monoclonal antibodies provide high specificity and affinity, crucial for targeted treatments in oncology and infectious diseases, but may elicit immune reactions limiting long-term use. Clinical effectiveness of humanized antibodies often surpasses murine monoclonal counterparts by minimizing adverse immune responses while maintaining therapeutic potency.
Immunogenicity: Risks and Considerations
Humanized antibodies exhibit reduced immunogenicity compared to murine monoclonal antibodies by incorporating human protein sequences, minimizing the risk of anti-drug antibody (ADA) development and adverse immune responses. Monoclonal antibodies derived fully from non-human species carry a higher immunogenicity risk, potentially causing hypersensitivity reactions, infusion-related side effects, and reduced therapeutic efficacy. Careful screening for immunogenic epitopes and monitoring ADA levels is essential to mitigate immune-related risks during therapeutic antibody development and clinical use.
FDA Approvals and Market Trends
FDA approvals reveal a growing preference for humanized antibodies in therapeutics, driven by their reduced immunogenicity compared to murine monoclonal antibodies. Market trends indicate accelerated adoption of humanized monoclonal antibodies in oncology and autoimmune diseases, with key products such as trastuzumab and pembrolizumab commanding significant market share. Continuous advancements in antibody engineering and increasing FDA approvals forecast sustained growth for humanized antibodies, challenging traditional murine monoclonal antibody dominance.
Cost and Accessibility Comparison
Humanized monoclonal antibodies typically incur higher production costs due to complex genetic engineering processes, impacting their overall pricing and limiting widespread accessibility in low-resource settings. Conventional monoclonal antibodies, while generally less expensive to produce, may present higher immunogenicity risks but offer more affordable treatment options in many healthcare systems. The choice between humanized and monoclonal therapies often balances efficacy, safety, and cost considerations, influencing patient access depending on regional healthcare funding and infrastructure.
Future Directions in Antibody Engineering
Future directions in antibody engineering emphasize enhancing the specificity and reducing the immunogenicity of both humanized and monoclonal antibodies through advanced techniques like bispecific and multispecific antibody development. Innovations such as antibody-drug conjugates (ADCs) and engineered Fc regions aim to improve therapeutic efficacy and safety profiles in clinical applications. Integration of artificial intelligence and high-throughput screening accelerates the design of next-generation antibodies with optimized stability, affinity, and functional diversity.
Humanized Infographic
 libterm.com
libterm.com