Complexing enhances molecular interactions by forming stable complexes that improve solubility, bioavailability, and stability of active compounds. This process is widely used in pharmaceuticals, cosmetics, and food industries to optimize product performance and efficacy. Explore the rest of the article to understand how complexing can benefit your formulations and applications.
Table of Comparison
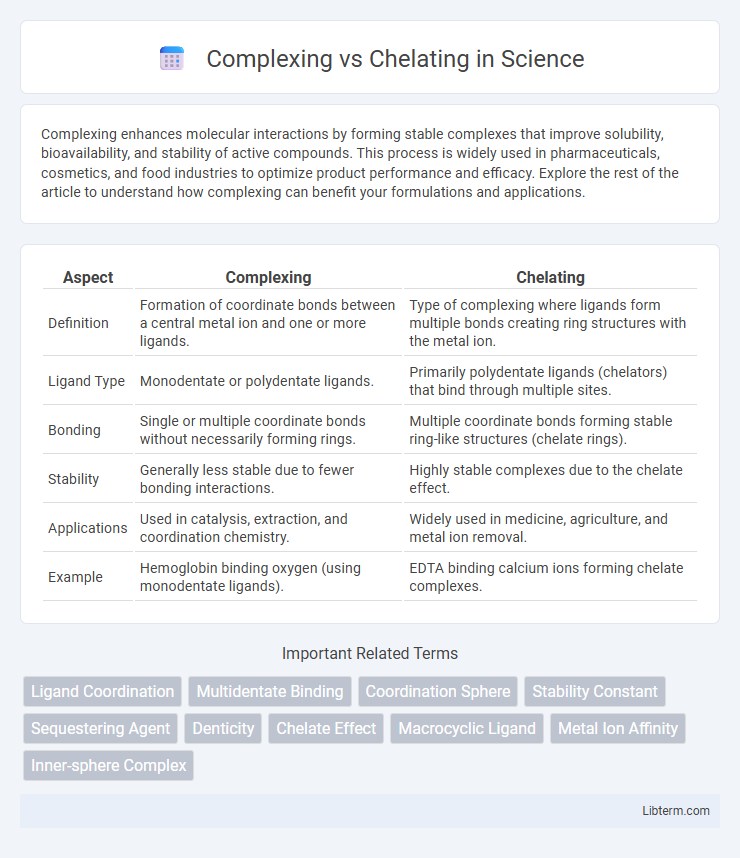
| Aspect | Complexing | Chelating |
|---|---|---|
| Definition | Formation of coordinate bonds between a central metal ion and one or more ligands. | Type of complexing where ligands form multiple bonds creating ring structures with the metal ion. |
| Ligand Type | Monodentate or polydentate ligands. | Primarily polydentate ligands (chelators) that bind through multiple sites. |
| Bonding | Single or multiple coordinate bonds without necessarily forming rings. | Multiple coordinate bonds forming stable ring-like structures (chelate rings). |
| Stability | Generally less stable due to fewer bonding interactions. | Highly stable complexes due to the chelate effect. |
| Applications | Used in catalysis, extraction, and coordination chemistry. | Widely used in medicine, agriculture, and metal ion removal. |
| Example | Hemoglobin binding oxygen (using monodentate ligands). | EDTA binding calcium ions forming chelate complexes. |
Understanding Complexing and Chelating: Key Differences
Complexing and chelating both involve the formation of bonds between a central metal ion and ligands, but chelating specifically refers to ligands that form multiple bonds creating a ring structure with the metal ion. The key difference lies in stability; chelates tend to form more stable complexes due to the multidentate ligand's ability to occupy multiple coordination sites, reducing the likelihood of metal ion dissociation. Understanding these differences is crucial in applications such as metal ion extraction, pharmaceuticals, and environmental remediation where stability and selectivity determine efficacy.
Definitions: What is Complexing?
Complexing refers to the process where molecules or ions, known as ligands, form coordinate bonds with a central metal ion, resulting in a coordination complex. This interaction involves the donation of electron pairs from ligands to the metal, stabilizing the metal ion in solution or solid state. Complexing plays a crucial role in fields like chemistry, biochemistry, and environmental science by influencing metal ion reactivity and bioavailability.
Definitions: What is Chelating?
Chelating refers to the process where a single ligand forms multiple bonds with a single metal ion, creating a stable ring-like complex called a chelate. This multi-dentate binding enhances the stability and solubility of metal ions in biological and chemical systems. Chelating agents like EDTA or citrate are widely used in medicine, agriculture, and industrial applications to control metal ion activity.
Chemical Mechanisms: How Complexing Works
Complexing involves the formation of coordination bonds between a central metal ion and surrounding ligands, where the ligands donate electron pairs to the metal, stabilizing it in solution. These interactions are typically reversible, with ligands attaching through lone pairs of electrons to form a coordination complex without altering the metal's oxidation state. The stability of the resulting complex depends on factors like ligand denticity, metal ion charge, and the geometry of the coordination sphere.
Chemical Mechanisms: How Chelation Works
Chelation involves the formation of a stable ring structure when a single ligand binds to a metal ion at multiple coordination sites, significantly enhancing the complex's stability. Unlike simple complexing, where ligands attach to a metal ion at one or more discrete points, chelation creates a cyclic compound that reduces the metal's reactivity and increases solubility. The multidentate nature of chelating agents such as EDTA allows them to sequester metal ions effectively by forming strong coordinate covalent bonds through donor atoms like nitrogen and oxygen.
Common Applications of Complexing Agents
Complexing agents are widely used in water treatment to prevent metal ion precipitation and improve solubility, especially for calcium and magnesium ions. In agriculture, these agents enhance nutrient availability by stabilizing micronutrients like iron and zinc in fertilizers, promoting efficient plant uptake. Industrially, complexing agents stabilize metal ions in dyeing and electroplating processes, ensuring consistent quality and preventing unwanted metal deposits.
Common Applications of Chelating Agents
Chelating agents are widely used in agriculture to enhance nutrient availability by forming stable complexes with metal ions, preventing their precipitation and facilitating plant uptake. Industrial applications include water treatment processes where chelating compounds such as EDTA bind heavy metals to prevent scaling and corrosion in boilers and cooling systems. In medicine, chelating agents serve as detoxifying substances to treat heavy metal poisoning by sequestering metals like lead or mercury and promoting their excretion from the body.
Advantages of Complexing vs Chelating
Complexing agents typically form reversible bonds with metal ions, offering advantages in processes requiring temporary stabilization or controlled release, unlike chelating agents which create more stable, often irreversible rings. This reversible nature of complexing facilitates easier regeneration and recyclability in industrial and environmental applications, improving cost efficiency and sustainability. Complexing agents also tend to have simpler molecular structures, enabling faster reaction kinetics and reduced toxicity compared to many chelating counterparts.
Limitations and Challenges of Each Method
Complexing agents often face limitations such as instability under varying pH levels and susceptibility to displacement by competing ions, which reduces their effectiveness in selective metal binding. Chelating agents, while providing stronger and more specific metal ion binding, can pose challenges including potential toxicity, environmental persistence, and the difficulty of controlling chelate biodegradability. Both methods require careful consideration of application context, metal ion specificity, and environmental impact to optimize their efficacy and safety.
Choosing Between Complexing and Chelating: Factors to Consider
Choosing between complexing and chelating agents depends primarily on the stability constant, specificity for metal ions, and the environmental conditions of use. Chelating agents form multiple bonds with a single metal ion, resulting in highly stable and selective complexes, ideal for applications requiring strong metal ion control such as in water treatment or medicine. Complexing agents, while generally forming less stable bonds, offer versatility and cost-effectiveness for processes where weaker or reversible metal ion interactions are acceptable, such as in certain industrial or agricultural contexts.
Complexing Infographic
 libterm.com
libterm.com