Absorption is the process by which substances are taken up into the body or another medium, often through membranes or tissues. This phenomenon is crucial in fields such as biology, chemistry, and environmental science for understanding how materials interact and transfer. Explore the rest of the article to discover how absorption impacts your daily life and various industries.
Table of Comparison
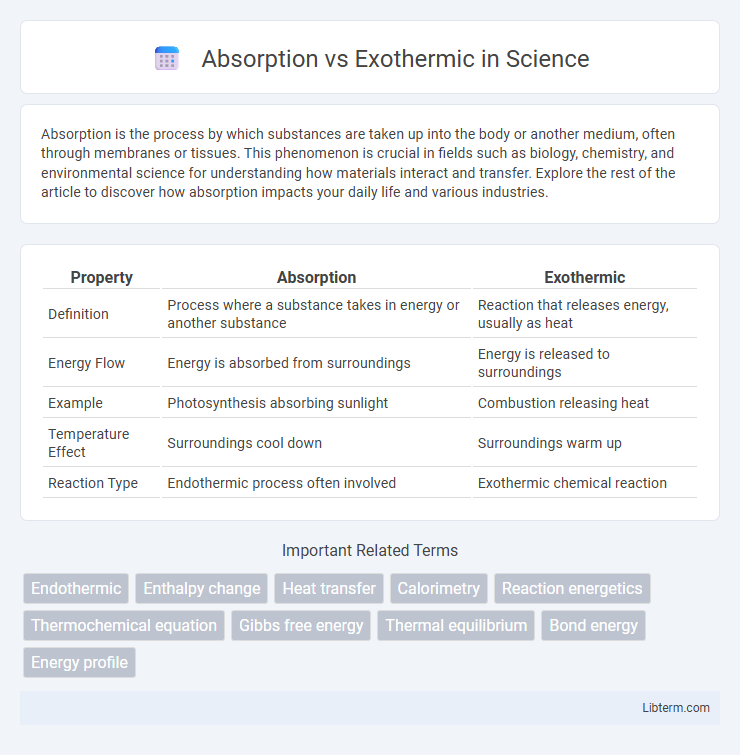
| Property | Absorption | Exothermic |
|---|---|---|
| Definition | Process where a substance takes in energy or another substance | Reaction that releases energy, usually as heat |
| Energy Flow | Energy is absorbed from surroundings | Energy is released to surroundings |
| Example | Photosynthesis absorbing sunlight | Combustion releasing heat |
| Temperature Effect | Surroundings cool down | Surroundings warm up |
| Reaction Type | Endothermic process often involved | Exothermic chemical reaction |
Understanding Absorption: A Scientific Overview
Absorption involves the uptake of molecules or energy by a substrate, frequently accompanied by a temperature change in the system. Unlike exothermic processes, where heat is released during a chemical reaction, absorption often entails energy transfer without necessarily emitting heat, as seen in phenomena like gas absorption in liquids or light absorption by pigments. Understanding absorption requires analyzing molecular interactions, energy states, and thermodynamic properties that govern how substances integrate or capture other entities at a microscopic level.
What Does Exothermic Mean? Core Concepts
Exothermic reactions release energy, primarily in the form of heat, to their surroundings, causing an increase in temperature. This energy release occurs when new bonds form with lower energy than the bonds broken, resulting in a net energy output. Understanding exothermic processes is crucial in fields like thermodynamics, chemical engineering, and environmental science, as they impact reaction spontaneity and energy management.
Key Differences Between Absorption and Exothermic Processes
Absorption involves the uptake of one substance by another, typically resulting in the physical or chemical incorporation of molecules, often accompanied by energy changes that can be endothermic or exothermic. Exothermic processes specifically release heat energy to the surroundings, indicating that energy is given off during the reaction. The key difference lies in absorption focusing on the mixing or uptake phenomenon, while exothermic describes the thermodynamic nature of a process releasing heat.
The Science Behind Absorption Reactions
Absorption reactions involve the uptake of a substance by another, often accompanied by the release or absorption of energy through molecular interactions. Exothermic absorption specifically refers to processes where energy is released as heat when substances, such as gases or liquids, are absorbed into solids or liquids, exemplified by the hydration of anhydrous salts. The science behind these reactions centers on changes in enthalpy and molecular bonding that stabilize the absorbed components, driving the energy exchanges that characterize absorption phenomena.
How Exothermic Reactions Release Energy
Exothermic reactions release energy by forming chemical bonds, where the energy released exceeds the energy required to break the initial bonds, resulting in a net energy release often in the form of heat or light. This energy emission occurs as the system transitions to a lower energy state, making exothermic processes critical in combustion, respiration, and many industrial applications. Heat generated from exothermic reactions can increase the temperature of the surroundings, driving various thermodynamic and kinetic phenomena.
Practical Applications of Absorption in Industry
Absorption processes are extensively utilized in industries such as chemical manufacturing, gas purification, and refrigeration, where they enable efficient separation and contamination control by selectively dissolving specific components. Unlike exothermic reactions, which release heat, absorption can be managed to optimize energy consumption and enhance process safety. Industrial applications benefit from absorption techniques in air pollution control, solvent recovery, and cooling systems due to their ability to handle large volumes of gases and liquids with minimal environmental impact.
Everyday Examples of Exothermic Reactions
Exothermic reactions release energy, often as heat or light, during chemical processes commonly seen in everyday life such as combustion in car engines and respiration in the human body. These reactions contrast with absorption processes where energy is stored or absorbed, as seen in photosynthesis and ice melting. Understanding exothermic reactions helps explain practical phenomena like the warmth generated by hand warmers and the heat produced when water freezes.
Comparing Energy Changes: Absorption vs Exothermic
Energy absorption involves the intake of heat, resulting in an increase in the system's internal energy, while exothermic processes release energy, causing a decrease in internal energy. In absorption, molecules gain energy to transition into a higher energy state, whereas exothermic reactions emit energy as molecules shift to a lower energy state. The thermodynamic difference is reflected in changes in enthalpy (DH), with absorption characterized by positive DH and exothermic reactions by negative DH values.
Environmental Impact: Absorption Versus Exothermic Processes
Absorption processes often involve capturing pollutants or carbon dioxide, reducing environmental emissions and promoting cleaner air. Exothermic reactions release heat, which can be harnessed for energy but may also contribute to thermal pollution if not managed properly. Effective utilization of absorption in environmental technologies minimizes harmful emissions, while controlling exothermic heat release mitigates ecological disruption.
Summary: Choosing Between Absorption and Exothermic Approaches
Absorption processes absorb heat, making them ideal for applications requiring cooling or heat dissipation, while exothermic reactions release heat, suitable for energy generation or heating purposes. Choosing between absorption and exothermic approaches depends on the system's thermal management needs, energy efficiency goals, and operational safety. Factors such as process scale, environmental impact, and material compatibility also influence the decision for industrial or chemical engineering applications.
Absorption Infographic
 libterm.com
libterm.com