Deposition is a crucial legal process where a witness provides sworn testimony outside of court, often used to gather evidence before a trial. This method helps clarify facts, assess witness credibility, and prepare both parties for litigation. Explore the article to understand how deposition might impact your legal case and what to expect during this procedure.
Table of Comparison
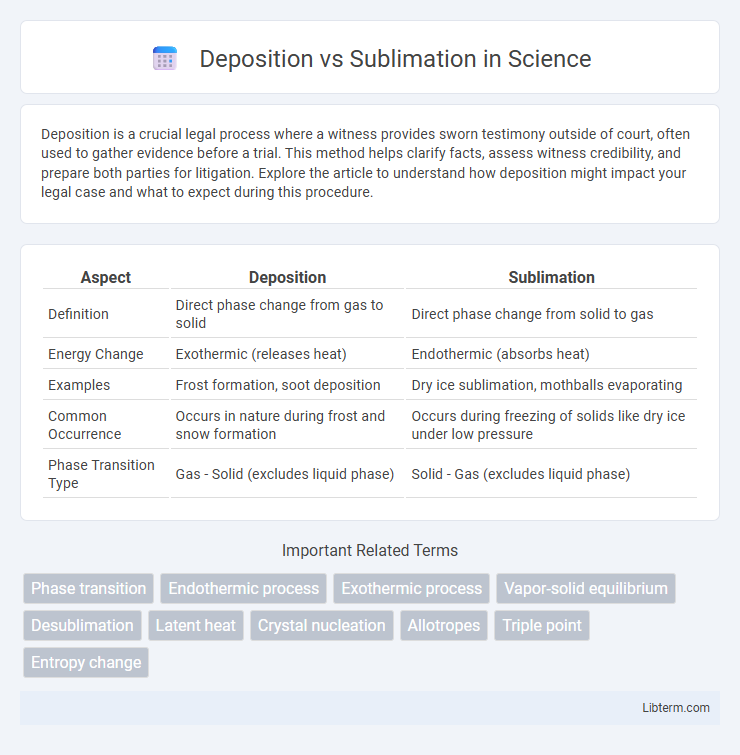
| Aspect | Deposition | Sublimation |
|---|---|---|
| Definition | Direct phase change from gas to solid | Direct phase change from solid to gas |
| Energy Change | Exothermic (releases heat) | Endothermic (absorbs heat) |
| Examples | Frost formation, soot deposition | Dry ice sublimation, mothballs evaporating |
| Common Occurrence | Occurs in nature during frost and snow formation | Occurs during freezing of solids like dry ice under low pressure |
| Phase Transition Type | Gas - Solid (excludes liquid phase) | Solid - Gas (excludes liquid phase) |
Understanding Deposition and Sublimation
Deposition is the phase transition where a gas changes directly into a solid without passing through the liquid state, commonly observed in frost formation. Sublimation is the opposite process, where a solid transforms directly into a gas, exemplified by dry ice turning into carbon dioxide vapor. Both processes bypass the liquid phase, highlighting unique energy and molecular interactions critical in meteorology, material science, and climate studies.
Defining Deposition: From Gas to Solid
Deposition is the phase transition in which a gas transforms directly into a solid without passing through the liquid state, often observed in the formation of frost on surfaces. This process occurs when gas molecules lose enough thermal energy to bond into a solid lattice, bypassing the liquid phase entirely. Common examples include the deposition of water vapor as ice crystals in cold environments and the manufacturing of thin films in semiconductor industries.
What is Sublimation? From Solid to Gas
Sublimation is the transition of a substance directly from the solid phase to the gas phase without passing through the intermediate liquid phase, commonly observed in substances like dry ice (solid carbon dioxide) and iodine crystals. This endothermic process occurs when molecules gain enough energy to overcome intermolecular forces, allowing them to vaporize directly. Sublimation plays a crucial role in various industrial applications, including freeze-drying and air purification.
The Science Behind Phase Transitions
Deposition and sublimation are phase transitions where matter changes directly between solid and gas states without passing through the liquid phase, governed by thermodynamic principles. Deposition occurs when gas molecules lose kinetic energy and aggregate into a solid, commonly observed in frost formation, while sublimation happens when a solid gains enough energy to transition into gas, such as dry ice vaporization. Both processes depend on pressure and temperature conditions described by the phase diagram and involve energy exchanges characterized by latent heat.
Key Differences Between Deposition and Sublimation
Deposition is the phase transition where a gas transforms directly into a solid without passing through the liquid state, while sublimation involves a solid changing directly into a gas. Key differences include energy exchange--deposition releases energy as an exothermic process, whereas sublimation requires energy absorption as an endothermic reaction. Deposition results in solid formation from vapor, commonly seen in frost formation, whereas sublimation is observed when dry ice vaporizes without melting.
Real-Life Examples of Deposition
Deposition occurs when water vapor transforms directly into ice, bypassing the liquid phase, as seen in frost formation on cold surfaces during autumn mornings. Another real-life example is the creation of snowflakes in clouds, where water vapor deposits onto particulate matter, forming intricate ice crystals. Industrial applications utilize deposition in the fabrication of thin films through chemical vapor deposition, essential for semiconductor manufacturing.
Everyday Instances of Sublimation
Sublimation occurs in everyday life when solid carbon dioxide, or dry ice, transforms directly into gas without becoming liquid, commonly used in fog machines for dramatic effects. Another example includes the freezing of mothballs that gradually shrink as the solid naphthalene sublimates into gas, helping repel insects. These instances highlight sublimation's practical applications in household and entertainment contexts, contrasting deposition, where gas turns directly into solid, like frost formation on cold surfaces.
Factors Influencing Deposition and Sublimation
Temperature and pressure significantly influence both deposition and sublimation processes by determining the phase transitions between gas and solid states. Surface characteristics and material properties affect nucleation rates and crystal formation during deposition, while heat transfer rates and ambient humidity impact sublimation intensity and duration. Environmental conditions such as air flow and contamination levels also play critical roles in controlling the efficiency and stability of these phase changes.
Applications in Industry and Technology
Deposition and sublimation are critical phase change processes used extensively in semiconductor manufacturing and material science. Deposition techniques, such as chemical vapor deposition (CVD) and physical vapor deposition (PVD), enable precise thin-film coatings essential for microelectronics, solar cells, and optical devices. Sublimation is exploited in freeze-drying pharmaceuticals, purifying heat-sensitive compounds, and fabricating advanced materials like graphene through vapor-phase sublimation processes.
Summary: Deposition vs. Sublimation in Nature and Science
Deposition and sublimation are phase changes where deposition involves a gas transforming directly into a solid, commonly seen in natural processes like frost formation on surfaces. Sublimation is the direct transition of a solid to a gas, exemplified by dry ice vaporizing without becoming liquid. Both processes play crucial roles in scientific applications such as cryogenics, meteorology, and material science, influencing phenomena like snowpack dynamics and vacuum deposition techniques.
Deposition Infographic
 libterm.com
libterm.com