Molecular science explores the structure, function, and interactions of molecules, which form the foundation of all living organisms and materials. Understanding molecular behavior enables advancements in medicine, chemistry, and nanotechnology. Discover how these insights can impact your knowledge and applications by reading more in the rest of the article.
Table of Comparison
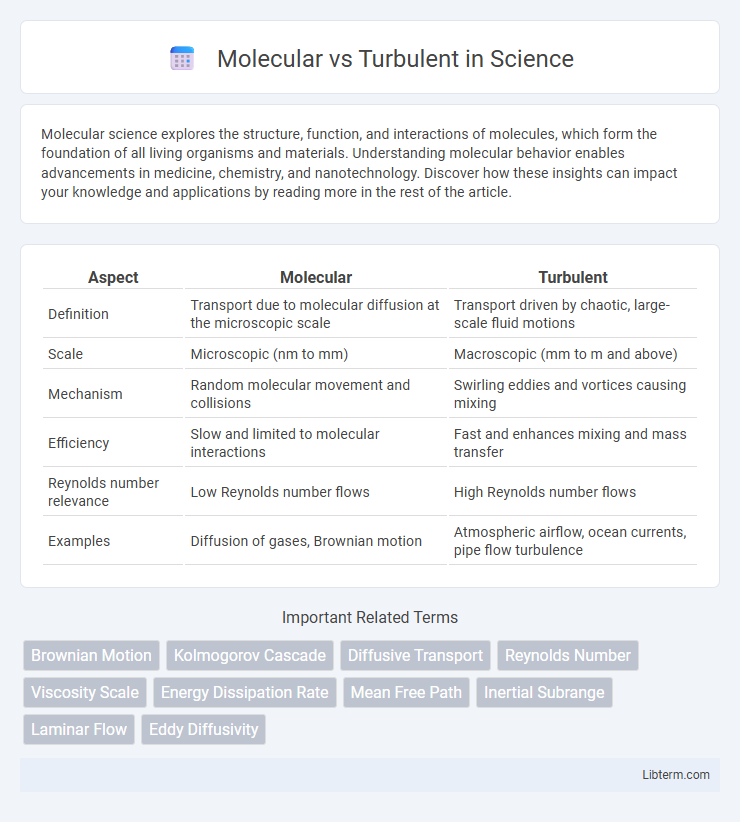
| Aspect | Molecular | Turbulent |
|---|---|---|
| Definition | Transport due to molecular diffusion at the microscopic scale | Transport driven by chaotic, large-scale fluid motions |
| Scale | Microscopic (nm to mm) | Macroscopic (mm to m and above) |
| Mechanism | Random molecular movement and collisions | Swirling eddies and vortices causing mixing |
| Efficiency | Slow and limited to molecular interactions | Fast and enhances mixing and mass transfer |
| Reynolds number relevance | Low Reynolds number flows | High Reynolds number flows |
| Examples | Diffusion of gases, Brownian motion | Atmospheric airflow, ocean currents, pipe flow turbulence |
Introduction to Molecular and Turbulent Phenomena
Molecular phenomena involve the random motion and interactions of individual molecules, governed by Brownian motion and diffusion principles at microscopic scales. Turbulent phenomena arise in fluid flows with high Reynolds numbers, characterized by chaotic, irregular eddies and vortices that enhance mixing and momentum transfer. Understanding the distinction between molecular viscosity and turbulent eddy viscosity is crucial for analyzing transport processes and energy dissipation in fluid dynamics.
Defining Molecular Motion
Molecular motion involves the random, microscopic movement of individual molecules driven by thermal energy, characterized by diffusion and Brownian motion. Turbulent motion, in contrast, encompasses chaotic, large-scale fluid flows with eddies and vortices that enhance mixing beyond molecular diffusion. Defining molecular motion is essential for understanding heat and mass transfer at the microscopic level, where molecular collisions dominate transport processes.
Understanding Turbulent Flow
Turbulent flow is characterized by chaotic fluid motion and eddies, significantly enhancing momentum and heat transfer compared to molecular diffusion. Unlike molecular flow, which relies on random molecular motion for transport, turbulent flow exhibits a complex hierarchy of vortices that increase mixing efficiency and accelerate mass transfer rates. Understanding turbulent flow is essential for optimizing engineering applications such as aerodynamic design, pipeline transport, and combustion processes where enhanced mixing improves system performance.
Fundamental Differences Between Molecular and Turbulent Processes
Molecular processes involve the random motion of individual molecules leading to diffusion and viscous effects at microscale levels, governed by molecular viscosity and Brownian motion. Turbulent processes, by contrast, are characterized by chaotic, multiscale eddies and vortices that enhance momentum, heat, and mass transfer far beyond molecular diffusion. The fundamental difference lies in the scale and mechanism: molecular transport is governed by molecular interactions, while turbulence arises from inertial instabilities and nonlinear fluid dynamics.
Mechanisms of Molecular Diffusion
Molecular diffusion occurs due to the random thermal motion of molecules, resulting in the transfer of mass at the microscopic scale driven by concentration gradients. This process is governed by Fick's laws, where the diffusion flux is proportional to the negative gradient of concentration. Unlike turbulent diffusion, molecular diffusion operates efficiently at low Reynolds numbers and laminar flows, with diffusion coefficients typically on the order of 10-9 to 10-5 m2/s for gases and liquids.
Characteristics of Turbulent Mixing
Turbulent mixing is characterized by chaotic, irregular fluid motion that enhances the dispersion of particles and scalars at multiple scales, resulting in rapid homogenization. Unlike molecular mixing, which relies on diffusion and occurs slowly at the microscopic level, turbulent mixing involves large eddies that break down into smaller vortices, increasing the interface area for mixing. This process significantly accelerates mass, momentum, and energy transfer in fluids, making it dominant in natural and industrial applications.
Scale and Energy Transfer in Molecular vs Turbulent Systems
Molecular systems operate at microscopic scales where energy transfer occurs primarily through molecular collisions and diffusion, leading to low Reynolds numbers and laminar flow patterns. Turbulent systems involve larger scales with chaotic and irregular fluid motion, characterized by high Reynolds numbers and enhanced mixing due to energy cascading from large eddies to smaller vortices. The key distinction lies in the scale-dependent mechanisms of energy dissipation, with molecular systems dominated by viscous forces and turbulent systems governed by inertial forces distributing kinetic energy across a wide range of scales.
Applications in Science and Engineering
Molecular and turbulent diffusion play crucial roles in various scientific and engineering applications, impacting heat and mass transfer processes. Molecular diffusion dominates in microscale systems like microfluidics and nanotechnology, where precise control over molecular transport is essential. Turbulent diffusion enhances mixing and reaction rates in large-scale processes such as combustion engines, chemical reactors, and environmental modeling, improving efficiency and performance.
Challenges in Modeling and Simulation
Modeling molecular versus turbulent flows presents significant challenges due to the vast differences in scale and behavior; molecular flows require quantum-level precision while turbulent flows involve complex, chaotic eddies spanning multiple scales. Capturing molecular interactions demands high computational cost and quantum mechanics frameworks, whereas turbulence necessitates sophisticated turbulence models like Large Eddy Simulation (LES) or Reynolds-Averaged Navier-Stokes (RANS) to approximate multi-scale vortices. Accurately bridging these regimes in simulations remains difficult, particularly in transitional flows where both molecular dynamics and turbulence influence fluid behavior, pushing the limits of current numerical methods and computational power.
Future Directions and Innovations
Future research in fluid dynamics emphasizes hybrid models integrating molecular-scale simulations with turbulent flow analyses to enhance predictive accuracy in complex environments. Advances in machine learning algorithms enable real-time turbulence modeling by capturing molecular interactions at unprecedented resolutions. Emerging innovations in quantum computing hold potential for revolutionizing the simulation of multiscale fluid behavior, bridging molecular dynamics and large-scale turbulent phenomena.
Molecular Infographic
 libterm.com
libterm.com