Substrate serves as the foundational layer on which materials, devices, or biological cultures are built or grown, playing a critical role in various industries from electronics to biotechnology. Its properties, such as composition, texture, and thermal conductivity, directly influence the performance and durability of the final product. Explore the rest of the article to understand how selecting the right substrate can optimize your project's success.
Table of Comparison
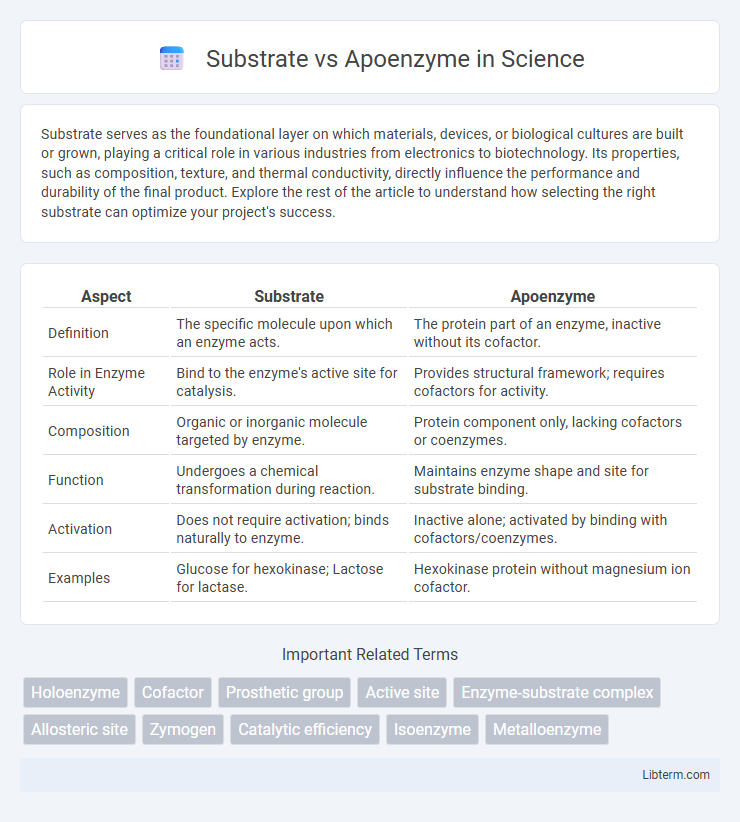
| Aspect | Substrate | Apoenzyme |
|---|---|---|
| Definition | The specific molecule upon which an enzyme acts. | The protein part of an enzyme, inactive without its cofactor. |
| Role in Enzyme Activity | Bind to the enzyme's active site for catalysis. | Provides structural framework; requires cofactors for activity. |
| Composition | Organic or inorganic molecule targeted by enzyme. | Protein component only, lacking cofactors or coenzymes. |
| Function | Undergoes a chemical transformation during reaction. | Maintains enzyme shape and site for substrate binding. |
| Activation | Does not require activation; binds naturally to enzyme. | Inactive alone; activated by binding with cofactors/coenzymes. |
| Examples | Glucose for hexokinase; Lactose for lactase. | Hexokinase protein without magnesium ion cofactor. |
Understanding Substrate and Apoenzyme: Key Definitions
Substrate refers to the specific molecule upon which an enzyme acts to catalyze a biochemical reaction, essential for processes like metabolism and cellular regulation. Apoenzyme denotes the protein portion of an enzyme, which remains inactive until combined with its necessary cofactor or coenzyme, forming the active holoenzyme. Understanding the roles of substrate and apoenzyme is crucial for grasping enzyme functionality and reaction mechanisms in biological systems.
Structural Differences: Substrate vs Apoenzyme
Substrate molecules are specific reactants that bind to an enzyme's active site, typically characterized by their small, well-defined molecular structures that complement the enzyme's shape. Apoenzymes, on the other hand, are the inactive protein components of enzymes lacking their required cofactor, exhibiting a more flexible and less structured conformation compared to the holoenzyme. Structural differences between substrates and apoenzymes fundamentally influence enzyme activity, with substrates fitting precisely into the active site, while apoenzymes require cofactor binding to achieve a fully functional, stable conformation.
Role of Substrate in Enzymatic Reactions
The substrate binds specifically to the active site of the enzyme, forming an enzyme-substrate complex that initiates the catalytic process. This precise interaction induces conformational changes in the enzyme, facilitating the conversion of the substrate into the product. The substrate's structural compatibility and concentration directly influence the reaction rate and enzymatic efficiency.
Apoenzyme: The Protein Component of Enzymes
Apoenzymes are the protein components of enzymes that require a non-protein cofactor to become catalytically active, contrasting with substrates, which are the reactants enzymes act upon. Apoenzymes provide the specific three-dimensional structure essential for substrate binding and catalytic function, enabling precise biochemical reactions. Understanding the role of apoenzymes is crucial for enzyme activity regulation, drug design, and metabolic pathway analysis.
Mechanism of Substrate Binding to Apoenzyme
Substrate binding to an apoenzyme involves a precise interaction where the substrate fits into the enzyme's active site, forming an enzyme-substrate complex essential for catalytic activity. This binding often induces conformational changes in the apoenzyme, stabilizing the transition state and enhancing catalytic efficiency. The specificity of substrate binding is dictated by the molecular complementarity between the substrate's structure and the apoenzyme's active site topology, driving the enzyme's biochemical function.
Substrate Specificity and Enzyme Function
Substrate specificity refers to the ability of an enzyme to selectively bind and catalyze a reaction with a particular substrate, a property largely determined by the enzyme's active site structure. The apoenzyme, which is the protein portion of an enzyme without its necessary cofactor, requires substrate recognition to initiate catalysis effectively. Substrates interact directly with the apoenzyme's active site, ensuring precise enzyme function and biochemical reaction fidelity.
Importance of Cofactors in Apoenzyme Activation
Cofactors play a critical role in apoenzyme activation by enabling the enzyme to achieve its functional conformation, which is essential for catalyzing biochemical reactions. Unlike substrates, which are molecules that enzymes act upon, cofactors are non-protein chemical compounds or metallic ions that bind to the apoenzyme, transforming it into an active holoenzyme. This interaction is vital for metabolic processes, as it ensures the precise catalytic activity necessary for cellular function.
Enzyme-Substrate Complex: Formation and Significance
The enzyme-substrate complex forms when the substrate binds precisely to the enzyme's active site, initiating a biochemical reaction. This interaction stabilizes the transition state, lowering the activation energy and ensuring substrate-specific catalysis. Understanding the enzyme-substrate complex is crucial for drug design and metabolic regulation, as it directly influences enzymatic efficiency and specificity.
Biological Examples of Substrate and Apoenzyme Interactions
Substrates like glucose bind specifically to apoenzymes such as hexokinase, facilitating phosphorylation during cellular respiration. In the interaction between lactose and b-galactosidase apoenzyme, substrate binding triggers conformational changes essential for lactose hydrolysis. Enzyme-substrate specificity is critical in processes like DNA replication, where substrates like nucleotides interact precisely with apoenzymes such as DNA polymerase to ensure accurate nucleotide addition.
Applications and Implications in Biotechnology and Medicine
Substrate specificity determines enzyme activity by binding directly to the active site, enabling precise metabolic reactions critical for drug development and metabolic engineering. Apoenzymes, as inactive protein components requiring cofactors, are fundamental in designing enzyme replacement therapies and biosensors. Understanding the interplay between substrates and apoenzymes enhances targeted biotechnology applications, such as synthetic biology and diagnostic tool innovation.
Substrate Infographic
 libterm.com
libterm.com