Metallic materials exhibit unique properties such as high electrical conductivity, durability, and malleability, making them essential in various industries from construction to electronics. Understanding the characteristics of different metals can help you choose the right material for your specific application. Explore the rest of the article to discover key insights and practical tips on working with metallic materials.
Table of Comparison
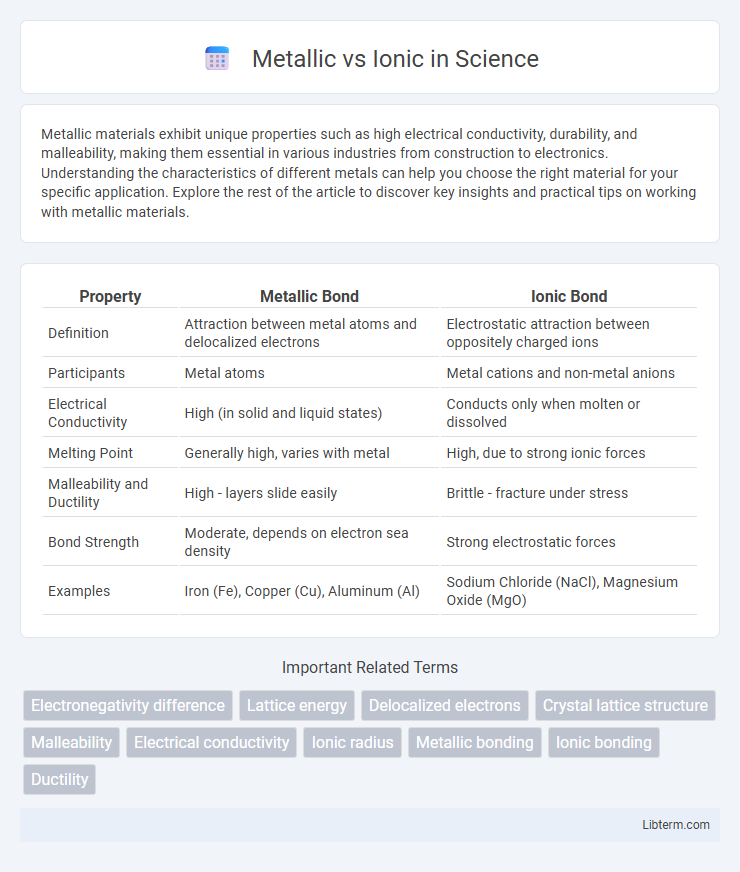
| Property | Metallic Bond | Ionic Bond |
|---|---|---|
| Definition | Attraction between metal atoms and delocalized electrons | Electrostatic attraction between oppositely charged ions |
| Participants | Metal atoms | Metal cations and non-metal anions |
| Electrical Conductivity | High (in solid and liquid states) | Conducts only when molten or dissolved |
| Melting Point | Generally high, varies with metal | High, due to strong ionic forces |
| Malleability and Ductility | High - layers slide easily | Brittle - fracture under stress |
| Bond Strength | Moderate, depends on electron sea density | Strong electrostatic forces |
| Examples | Iron (Fe), Copper (Cu), Aluminum (Al) | Sodium Chloride (NaCl), Magnesium Oxide (MgO) |
Introduction to Metallic and Ionic Bonds
Metallic bonds occur when metal atoms share a "sea" of delocalized electrons, allowing for high electrical conductivity and malleability. Ionic bonds form through the electrostatic attraction between positively charged cations and negatively charged anions, typically between metal and non-metal atoms. These fundamental bonding types significantly influence the physical properties and chemical behaviors of materials.
Fundamental Differences Between Metallic and Ionic Bonds
Metallic bonds involve the sharing of free electrons among a lattice of metal atoms, creating a "sea of electrons" that facilitates electrical conductivity and malleability. Ionic bonds form between positively charged cations and negatively charged anions through electrostatic attraction, resulting in rigid, high-melting-point crystalline structures. The fundamental distinction lies in electron behavior: metallic bonds feature delocalized electrons, while ionic bonds exhibit electron transfer and localization.
Structure and Formation of Metallic Bonds
Metallic bonds form through the delocalization of valence electrons, creating a lattice of positively charged metal ions surrounded by a "sea of electrons" that facilitates electrical conductivity and malleability. This structure contrasts with ionic bonds, where electrons are transferred between atoms, resulting in a rigid crystalline lattice of alternating cations and anions. The metallic bonding's electron mobility contributes to metal's ductility and thermal conductivity, characteristics absent in the fixed electron positions of ionic compounds.
Structure and Formation of Ionic Bonds
Ionic bonds form through the electrostatic attraction between positively charged cations and negatively charged anions, creating a crystalline lattice structure that maximizes stability. This lattice arrangement results in high melting and boiling points due to strong ionic interactions within the rigid, three-dimensional framework. In contrast, metallic bonds consist of a sea of delocalized electrons surrounding metal cations, facilitating conductivity and malleability not present in ionic compounds.
Electrical Conductivity: Metallic vs Ionic Compounds
Metallic compounds exhibit high electrical conductivity due to the presence of free-moving delocalized electrons within the metal lattice, enabling efficient charge transfer. In contrast, ionic compounds conduct electricity only when molten or dissolved in water, as their rigid ionic lattice restricts ion mobility in the solid state. This fundamental difference in electrical conductivity arises from the electron mobility in metallic bonding versus the ionic mobility in ionic bonding environments.
Physical Properties: Hardness, Malleability, and Brittleness
Metallic bonds create materials with high malleability and ductility due to the sea of delocalized electrons, allowing atoms to slide past each other without breaking. Ionic compounds exhibit high hardness but are brittle, as the electrostatic forces between oppositely charged ions resist deformation but cause the lattice to fracture under stress. The contrasting physical properties stem from metallic bonding's electron mobility versus ionic bonding's rigid lattice structure.
Melting and Boiling Points Comparison
Metallic bonds exhibit a sea of delocalized electrons, resulting in high melting and boiling points due to strong electrostatic attractions between metal cations and free electrons. Ionic compounds possess even higher melting and boiling points because the electrostatic forces between oppositely charged ions form rigid crystal lattices, requiring significant energy to break. The melting and boiling points of ionic substances typically surpass those of metals, reflecting the strength of ionic bonds compared to metallic bonding.
Solubility Differences in Water and Other Solvents
Metallic compounds exhibit poor solubility in water due to their lattice of delocalized electrons, whereas ionic compounds typically dissolve well in water because polar water molecules stabilize the separated ions. Ionic solids dissociate into cations and anions, allowing for high solubility in polar solvents but low solubility in nonpolar solvents like hexane. In contrast, metallic substances maintain strong metallic bonds and generally exhibit insolubility in both polar and nonpolar solvents.
Real-World Examples and Applications
Metallic bonds are exemplified by copper and aluminum, which are widely used in electrical wiring and construction due to their excellent conductivity and malleability. Ionic compounds like sodium chloride and calcium carbonate find crucial applications in daily life, including table salt for seasoning and calcium carbonate in cement and antacid tablets. These real-world applications demonstrate metallic substances' strength and ductility, while ionic compounds' high melting points and solubility enable diverse industrial and household uses.
Conclusion: Choosing Between Metallic and Ionic Compounds
Choosing between metallic and ionic compounds depends on desired properties such as conductivity, malleability, and melting point. Metallic compounds excel in electrical conductivity and ductility due to delocalized electrons, while ionic compounds offer high melting points and strong electrostatic forces resulting in brittleness. Application-specific requirements dictate whether the flexibility and conductivity of metallic bonds or the hardness and thermal stability of ionic bonds are more advantageous.
Metallic Infographic
 libterm.com
libterm.com