Conformational isomers are molecules that differ by rotation around a single bond, leading to distinct spatial arrangements without breaking chemical bonds. These variations significantly impact the physical and chemical properties of compounds, influencing reactivity, stability, and biological activity. Explore the rest of the article to understand how conformational isomerism affects your approach to molecular design and analysis.
Table of Comparison
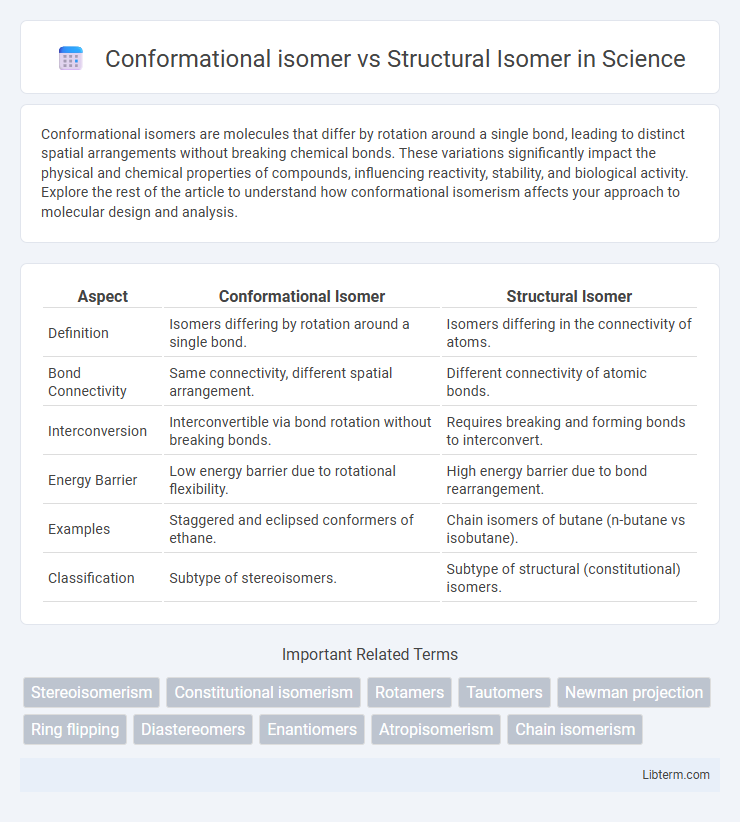
| Aspect | Conformational Isomer | Structural Isomer |
|---|---|---|
| Definition | Isomers differing by rotation around a single bond. | Isomers differing in the connectivity of atoms. |
| Bond Connectivity | Same connectivity, different spatial arrangement. | Different connectivity of atomic bonds. |
| Interconversion | Interconvertible via bond rotation without breaking bonds. | Requires breaking and forming bonds to interconvert. |
| Energy Barrier | Low energy barrier due to rotational flexibility. | High energy barrier due to bond rearrangement. |
| Examples | Staggered and eclipsed conformers of ethane. | Chain isomers of butane (n-butane vs isobutane). |
| Classification | Subtype of stereoisomers. | Subtype of structural (constitutional) isomers. |
Introduction to Isomerism
Isomerism refers to compounds with the same molecular formula but different arrangements of atoms, resulting in distinct properties. Conformational isomers, or conformers, differ by rotation around single bonds without breaking chemical bonds, exemplified by staggered and eclipsed forms of ethane. Structural isomers, also called constitutional isomers, vary in the connectivity of atoms, leading to different functional groups or bonding patterns, such as butanol and diethyl ether.
Defining Structural Isomers
Structural isomers, also known as constitutional isomers, are molecules with the same molecular formula but different connectivity of atoms, resulting in distinct chemical properties. Unlike conformational isomers, which differ only in spatial arrangement due to rotation around single bonds, structural isomers have unique covalent bond linkages. Examples include chain isomers, position isomers, and functional group isomers, each exhibiting different physical and chemical characteristics.
Understanding Conformational Isomers
Conformational isomers are molecules that differ by rotation around a single bond, existing in different spatial arrangements without breaking covalent bonds. These isomers can rapidly interconvert, leading to variations in stability, energy levels, and steric interactions, crucial for understanding molecular flexibility and reactivity. Unlike structural isomers, which have different covalent connectivity, conformational isomers maintain the same atomic connectivity, impacting physical and chemical properties subtly but significantly.
Key Differences Between Structural and Conformational Isomers
Structural isomers differ in the connectivity of atoms, resulting in distinct molecular formulas and unique physical and chemical properties, while conformational isomers have the same connectivity but differ in spatial orientation due to rotation around single bonds. Structural isomers include chain, positional, and functional group isomers, whereas conformational isomers primarily involve staggered, eclipsed, and gauche conformations in molecules like alkanes. The key distinction lies in structural isomers exhibiting different atomic linkages, whereas conformational isomers interconvert through bond rotations without breaking chemical bonds.
Types of Structural Isomerism
Structural isomerism includes various types such as chain isomerism, where compounds differ in carbon chain arrangement; positional isomerism, involving different positions of functional groups on the same carbon skeleton; and functional group isomerism, where isomers contain different functional groups altogether. Conformational isomerism differs by referring to different spatial orientations of the same molecule, resulting from rotation around single bonds without breaking chemical bonds. Understanding these distinctions is critical in fields like organic chemistry and pharmaceuticals, where molecular structure impacts properties and reactivity.
Types of Conformational Isomerism
Conformational isomers, also known as conformers, differ by rotation around single bonds without breaking any bonds, primarily including staggered, eclipsed, gauche, and anti forms in alkanes. Structural isomers have different connectivity of atoms, leading to distinct compounds like chain isomers, positional isomers, and functional group isomers. Types of conformational isomerism specifically involve rotations that alter torsional strain and steric hindrance, significantly impacting molecular stability and reactivity.
Examples of Structural Isomers
Structural isomers differ in the connectivity of their atoms, leading to variations in molecular formulas and properties, while conformational isomers arise from rotations around single bonds without breaking covalent bonds. Examples of structural isomers include butanol and isobutanol, which have the same molecular formula (C4H10O) but different connectivity, and glucose and fructose, both C6H12O6 but differing in carbonyl group placement. These variations impact chemical reactivity and physical characteristics, illustrating the significance of structural isomerism in organic chemistry.
Examples of Conformational Isomers
Conformational isomers, also known as conformers, differ by rotation around a single bond without breaking any bonds, exemplified by the staggered and eclipsed forms of ethane. Cyclohexane displays prominent conformational isomers such as the chair, boat, and twist-boat conformations, which interconvert by ring-flipping. In contrast, structural isomers have different connectivity of atoms, exemplified by butanol and isobutanol, which are distinct compounds with different bonding arrangements.
Significance in Chemical Properties and Reactivity
Conformational isomers, resulting from rotations around single bonds, exhibit differences primarily in spatial arrangement, influencing chemical properties such as steric strain and reaction rates without altering connectivity. Structural isomers, characterized by distinct bonding patterns and connectivity of atoms, display significant variations in chemical reactivity, physical properties, and functional group behavior. Understanding these distinctions is crucial for predicting molecular interactions, stability, and pathways in synthesis and enzymatic reactions.
Conclusion: Choosing the Right Isomerism for Applications
Conformational isomers differ in the rotation around single bonds, making them crucial in understanding molecular flexibility and dynamic behavior, especially in drug design and protein folding. Structural isomers, which vary in the connectivity of atoms, are essential for identifying distinct compounds with different chemical and physical properties in synthetic chemistry and material science. Selecting the appropriate type of isomerism depends on the application requirements, with conformational isomers favored for studying dynamic processes and structural isomers preferred for exploring distinct molecular frameworks.
Conformational isomer Infographic
 libterm.com
libterm.com