Combustion is a chemical process that rapidly oxidizes a fuel, releasing heat and light energy. Efficient combustion is essential for energy production, engine performance, and reducing harmful emissions. Discover how optimizing combustion can improve your energy use by reading the rest of the article.
Table of Comparison
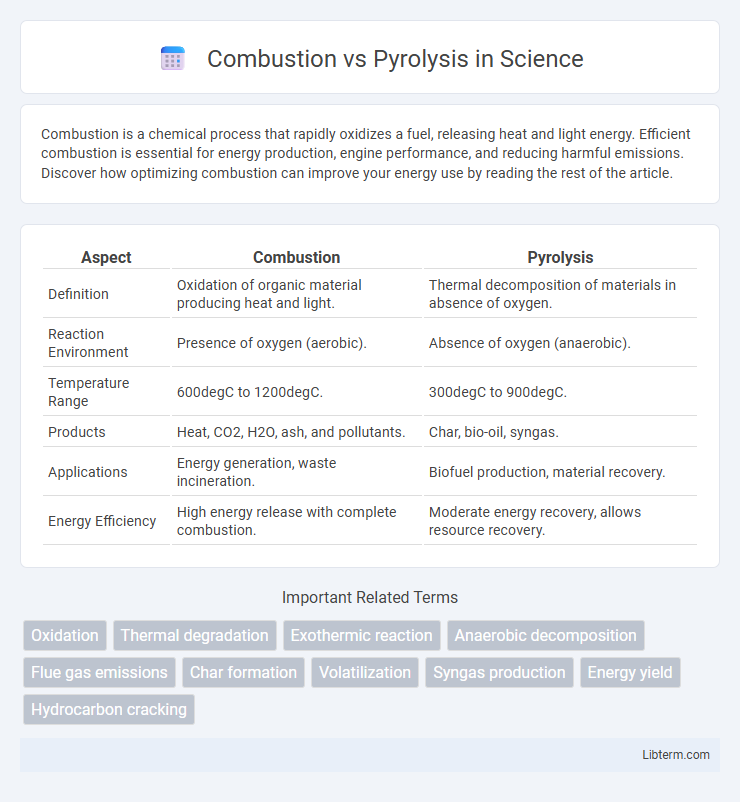
| Aspect | Combustion | Pyrolysis |
|---|---|---|
| Definition | Oxidation of organic material producing heat and light. | Thermal decomposition of materials in absence of oxygen. |
| Reaction Environment | Presence of oxygen (aerobic). | Absence of oxygen (anaerobic). |
| Temperature Range | 600degC to 1200degC. | 300degC to 900degC. |
| Products | Heat, CO2, H2O, ash, and pollutants. | Char, bio-oil, syngas. |
| Applications | Energy generation, waste incineration. | Biofuel production, material recovery. |
| Energy Efficiency | High energy release with complete combustion. | Moderate energy recovery, allows resource recovery. |
Introduction to Combustion and Pyrolysis
Combustion is a chemical process characterized by the rapid oxidation of a fuel, producing heat, light, and combustion byproducts such as carbon dioxide and water vapor. Pyrolysis involves the thermal decomposition of organic materials in the absence of oxygen, generating biochar, syngas, and bio-oil. Both processes play crucial roles in energy production and waste management but differ fundamentally in oxygen availability and reaction dynamics.
Defining Combustion: Fundamentals and Process
Combustion is a high-temperature exothermic oxidation reaction between a fuel and an oxidant, usually oxygen, resulting in the release of heat and light. This process involves rapid chemical reactions that convert chemical energy into thermal energy, producing combustion products such as carbon dioxide, water vapor, and heat. Efficient combustion requires proper mixing of fuel and oxidant, sufficient temperature, and the presence of an ignition source to sustain the reaction.
Understanding Pyrolysis: Mechanism and Principles
Pyrolysis is a thermochemical decomposition process occurring in the absence of oxygen, where organic materials break down into smaller molecules due to high temperatures, typically between 350degC and 700degC. The mechanism involves complex chemical reactions such as bond cleavage, free radical formation, and molecular rearrangement, resulting in the production of gases, bio-oil, and char. Unlike combustion, pyrolysis focuses on controlled thermal degradation without oxidation, making it crucial for biofuel production, waste management, and materials recycling.
Key Differences Between Combustion and Pyrolysis
Combustion is a chemical process involving the rapid oxidation of a fuel with oxygen, producing heat, light, carbon dioxide, and water, whereas pyrolysis is the thermal decomposition of organic material in the absence of oxygen, yielding char, tar, and syngas. Combustion occurs at high temperatures with sufficient oxygen, resulting in complete fuel oxidation and energy release, while pyrolysis operates at moderate temperatures with limited or no oxygen, focusing on breaking chemical bonds to generate valuable intermediate products. The key differences lie in the presence of oxygen, reaction temperature, and the type of end products formed during each process.
Chemical Reactions: Combustion vs Pyrolysis
Combustion involves chemical reactions where organic compounds react rapidly with oxygen, producing carbon dioxide, water, heat, and light through exothermic oxidation processes. Pyrolysis occurs in the absence of oxygen, breaking down complex organic molecules into simpler hydrocarbons, gases, and char via thermal decomposition. While combustion releases energy through oxidation, pyrolysis transforms materials chemically without direct oxygen interaction, emphasizing different chemical pathways.
Energy Output and Efficiency Comparison
Combustion involves complete oxidation of fuel, producing higher energy output per unit of feedstock with typical thermal efficiencies ranging from 30% to 40%. Pyrolysis operates under anaerobic conditions, breaking down organic material into bio-oil, gas, and char, with energy conversion efficiencies generally between 50% and 70%, depending on feedstock and reactor design. While combustion releases immediate energy primarily as heat, pyrolysis offers more versatile energy products and can achieve higher overall energy utilization through subsequent processing.
Environmental Impact: Emissions and By-products
Combustion generates significant greenhouse gases such as CO2 and pollutants like NOx and particulate matter, contributing to air pollution and climate change. Pyrolysis produces fewer harmful emissions by thermally decomposing organic material in an oxygen-free environment, resulting in biochar, syngas, and bio-oil as valuable by-products. These by-products can be utilized for soil enhancement and renewable energy, presenting a lower environmental footprint compared to traditional combustion processes.
Industrial Applications of Combustion and Pyrolysis
Industrial combustion is widely used for energy generation in power plants, steel manufacturing, and cement production due to its efficiency in converting fossil fuels into heat. Pyrolysis finds key applications in waste management and biochar production, enabling the thermal decomposition of organic materials at high temperatures in oxygen-free environments to generate valuable chemicals and fuels. Both processes contribute significantly to sustainable industrial practices by optimizing resource recovery and reducing environmental impact.
Advantages and Limitations of Each Process
Combustion offers rapid energy release and is widely used for power generation due to its high efficiency in converting fuel to heat, but it produces significant emissions including CO2 and NOx, contributing to environmental pollution. Pyrolysis enables the thermal decomposition of organic material in an oxygen-free environment, yielding valuable products like bio-oil, syngas, and char with lower pollutant output, yet it requires precise temperature control and has slower reaction rates, limiting scalability. Both processes play crucial roles in waste management and renewable energy, with combustion excelling in immediate energy output and pyrolysis favored for material recovery and reduced environmental impact.
Future Trends in Combustion and Pyrolysis Technologies
Future trends in combustion and pyrolysis technologies emphasize enhanced energy efficiency and reduced emissions through advanced catalyst development and optimized reactor designs. Integration of artificial intelligence and machine learning facilitates real-time process control, improving operational performance and environmental compliance. Emerging sustainable feedstocks and carbon capture solutions are driving innovations toward cleaner, circular economy-oriented combustion and pyrolysis systems.
Combustion Infographic
 libterm.com
libterm.com